| Oracle® Argus Insight User's Guide, Release 7.0 E22886-01 |
|
 Previous |
 Next |
Argus Insight provides built-in Standard Reports which can be run on Active Case Series to analyze your company's safety, workflow, and product data.
Although Standard Reports are predefined reports, you can define pre-filters before generating a Standard Report to have the report output display information only about specific type of cases. Pre-filters let you narrow down the Case Series further so that the system runs the report only on those cases that confirm to the pre-filter criteria. For example, your Case Series might consist of cases that were reported in all the countries for a particular product.
However, you may only wish to see information about those cases in your report that were reported in the United States. In this case, you can specify a pre-filter to have the system display only those cases in the report output that were reported in the United States.
|
Note: Since a Case Series might become obsolete each time the datamart is refreshed by running an ETL, you may need to generate the Case Series again before generating a Standard Report.Alternatively, you can directly associate aquery (QBE, Filter, or Advanced Condition) to a Standard Report to avoid manual generation of Case Series. See the Associating a QBE with a Report, Associating a Value Set with a Report, and Associating an Advanced Condition with a Report topics for details. |
Before using Argus Insight to generate the Standard Reports, configure the browser as defined in the Argus Insight Installation Guide.
Before generating reports, you should be aware of information regarding the following:
Event Level Reporting
Report Scheduling
Use the following procedure to access the Reports page for any category. For instance, if you select Compliance Reports, execute the following steps:
Select the Case Series Reports > Standard Reports >Compliance. The Standard Reports page displays a list of all the standard Compliance reports. The description of a report is displayed next to its name.
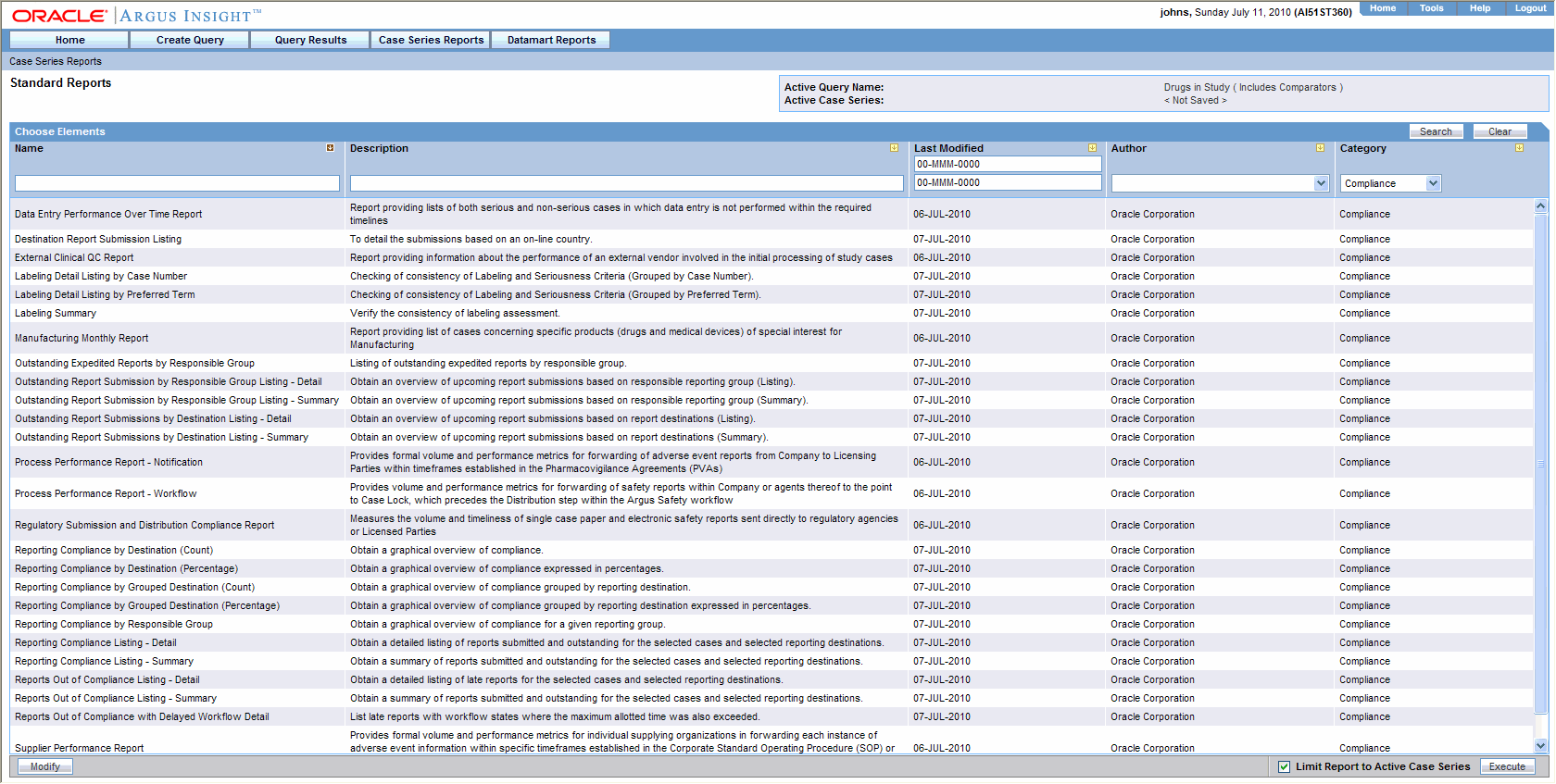
Select the report that you want to generate.
Select the Limit to Active Case Series checkbox to generate the report on the Active Case Series.
|
Note: Select the Limit to Active Case Series checkbox before generating the report. This prevents the report from querying the entire datamart and slowing down the report output generation. Use the following procedure to view the Active Case Series, select Query Results > Case Series > Active in Argus Insight. If you want to make another Case Series active, select Query Results > Case Series > Library in Argus Insight. In the Case Series Library page that appears, check the Case Series you want to make active and click Make Active. |
Click Execute to generate the report. A new browser window displays the pre-filter options in the Report Filter page. The following pre-filter elements may be available depending on the type of information the report displays.
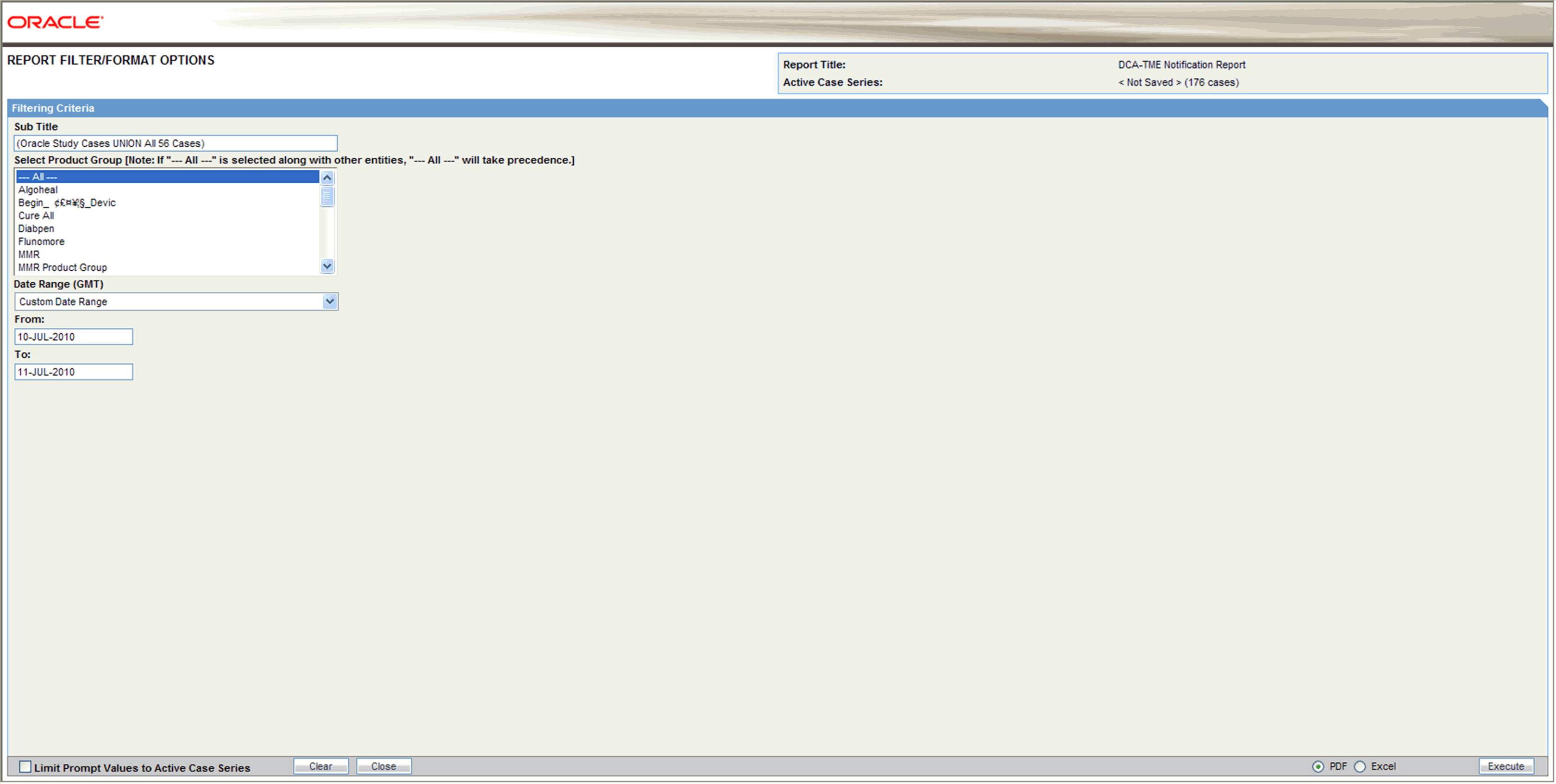
List box - this element lets you filter the report output by specifying a single value from the list; for example, the country of incidence, a regulatory authority, or a pre-defined date range. List boxes may also contain options for grouping the report output; for example, grouping by age group or product name
Multiple selection lists - this element lets you filter the report output by multiple values you select from the list; for example, license countries or report types.
|
Note: If All is selected along with other entities for a category, then all the items under that category are searched. |
Text boxes - this element lets you filter the report output by specifying a numerical value for timelines or dates for date ranges. All the report pre-filter pages contain a text box for specifying a sub-title for the report
The Pre-filter page can display values in Prompts. By default, prompts on the Pre-filter page display values from LM tables.
By default, a checkbox called Limit Prompts Values to Active Case Series is displayed on the Pre-filter page. You can check this checkbox to generate pre-filter prompts and view values limited to the cases in the case series. This impacts only those prompts which are populated from case tables and corresponding LM tables exist for that prompt.
Custom prompts created by the user for ad-hoc reporting do not have the option of displaying values in prompts from LM tables. Custom prompts always display data as per the conditions (SQL) that were defined during the creation of the prompts.
Specify the pre-filter options, as appropriate.
Select the PDF or Excel option button to specify the report output format.
Click Execute in the Report Filter page. The system generates the report output and displays it in the selected format. You can print this report or save it to the system drive, if required.
Close the new browser window to return to the Standard Reports page.
Currently, Insight Reports are focused on cases. This permits event level report in Insight Report output.
Extended reporting in the standard reports enables the user to produce event-level reports. To produce an event-level report, the user must click the "Limit to events within Query Criteria" checkbox shown in the following illustration.

The event group information is populated from the Event Groups.
The user can select multiple Event Groups.
If the user selects the Event groups, the system limits the output to only the Events selected in the Event Group definition.
If the user clicks "Limit to Events within the Query Criteria," the system limits report output to the Events chosen in the report output query criteria.
This applies to all queries executed from QBE, Filters, or Advanced Conditions
This applies for Event Terms for the entire hierarchy (i.e., SOC, HLGT, HLT, PT, LLT for the MedDRA Coded events only).
This cannot be executed with hard-coded SQL queries.
This feature cannot be used with a case series from Safety.
This feature cannot be used with an imported case series.
If the user uses a power query to generate a case series, the following terms are respected as the event query in the reports:
From QBE - Events tab - Event Coding
System Organ Class (SOC)
High Level Group Term
High Level Term
Preferred Term
Lower Level Term
From Filters - Event Information
Event Term -
i. SOC
ii. HLGT
iii. HLT
iv. PT
v. LLT
Preferred Term
From Advanced Conditions -
EVENTS:Event Information Event Body System Code
EVENTS:Event Information System Organ Class (SOC)
EVENTS:Event Information Event High Level Group Term Code
EVENTS:Event Information High Level Group Term
EVENTS:Event Information Event High Level Term Code
EVENTS:Event Information High Level Term
EVENTS:Event Information Preferred Term
EVENTS:Event Information Preferred Term Code
EVENTS:Event Information Event Low Level Term
EVENTS:Event Information Lower Level Term
EVENTS:Event Information Event Included Term Code
EVENTS:Event Information Event SMQ (Broad)
EVENTS:Event Information Event SMQ (Narrow)
EVENTS:Primary Event Event SMQ (Broad)
EVENTS:Primary Event Event SMQ (Broad)
This feature is available in the following reports:
Event term frequency listing by HLGT
Event term frequency listing by HLT
Event term frequency listing by PT
Event term frequency listing by SOC
AE count tabulation (case causality)
Report Scheduling comprises the following:
Scheduling Multiple Reports Against a Single Query
Scheduling a Report without a Query
The system enables users to schedule multiple reports in a single query. A set of reports run on a weekly or periodic basis can be scheduled with the same report and run as a package.
On clicking the Associate button from the query library, only one report is selected. If a different report is chosen for the query, the system prompts the user is to change. The system permits the user to associate many reports with a single query.
The user can select any query from any library (QBE/Filters/AC) and click Associate.
The user can use the Query Library and the Associated Query Library to schedule an association.
Since multiple reports can be associated with a single query on these Query pages, the Execute button does not open any pre-filters page after generating the case series.
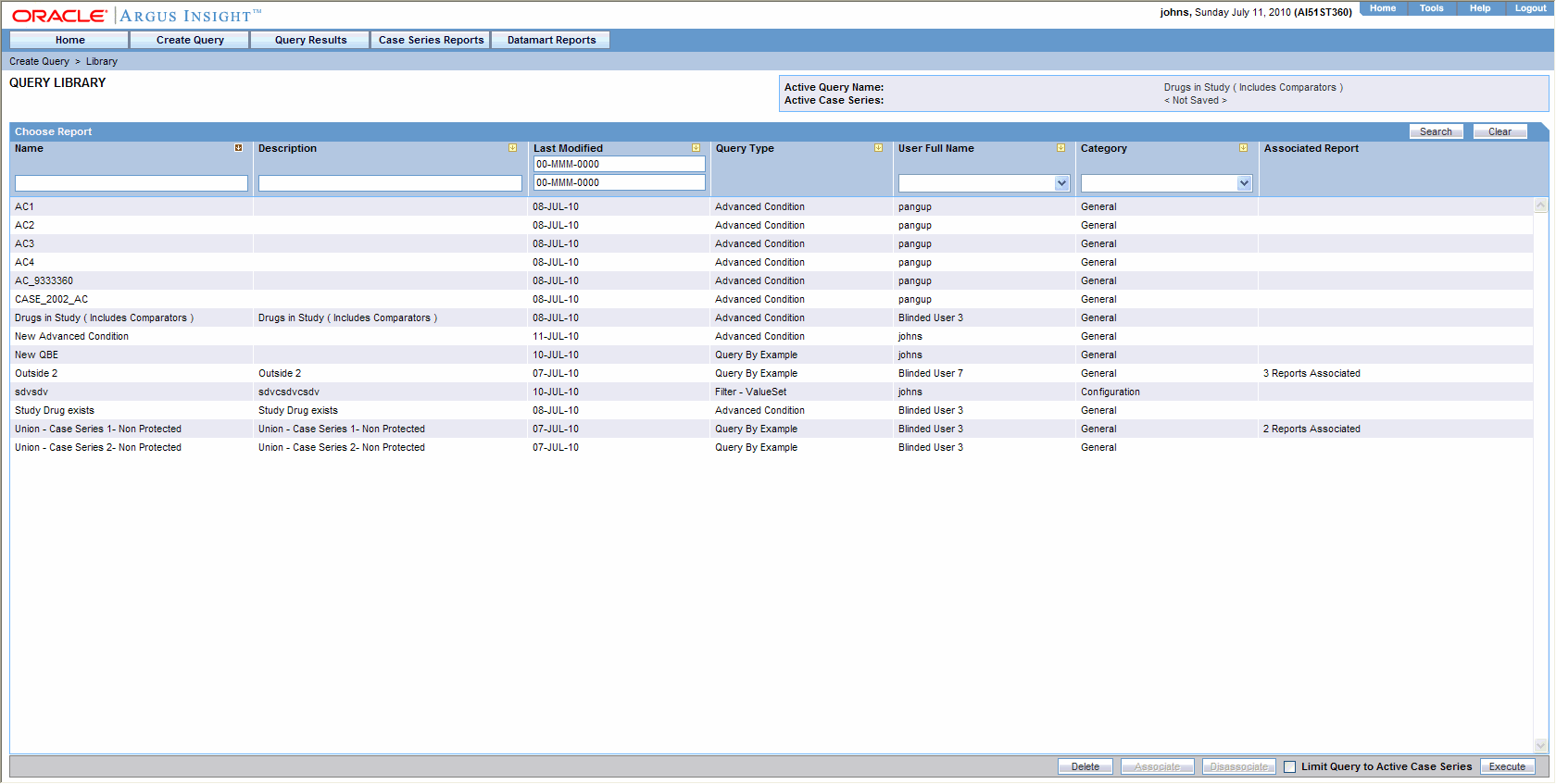
When you select a query and click Associate, the Scheduled Report Groups window is displayed.
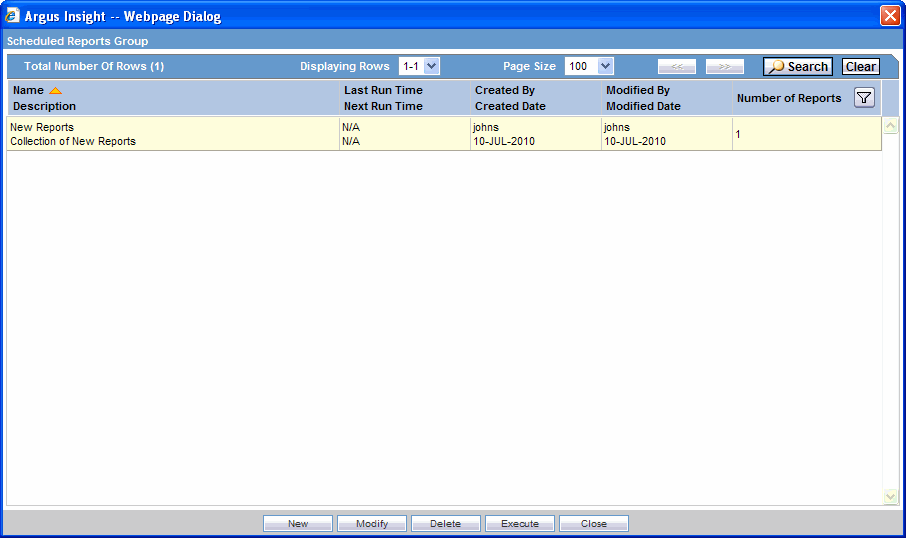
This window includes the following features:
This window displays information about all the report groups scheduled by the logged-in user.
Sorting and Search functionality is available for all columns.
Pagination is also available.
Each group can contain one or more reports. "Group" means that all the reports are scheduled at the same time.
This window has the following buttons:
New: Redirects the user to the Report Scheduling page with blank values. The system presents the context menu to enable the user to choose Report Association Only or for Scheduling.
Modify: Redirects the user to the Report Scheduling page and enables the user to modify an existing scheduled report group.
Delete: Deletes a selected scheduled report group.
Execute: Closes the window and executes the query in parent window to show case series and launch pre-filter for the selected report on this page. This button is enabled on if the scheduled report group has a single report.
Close: Closes the window without any other action.
The system opens the Schedule Information page when the user selects Association Only from the drop-down list.
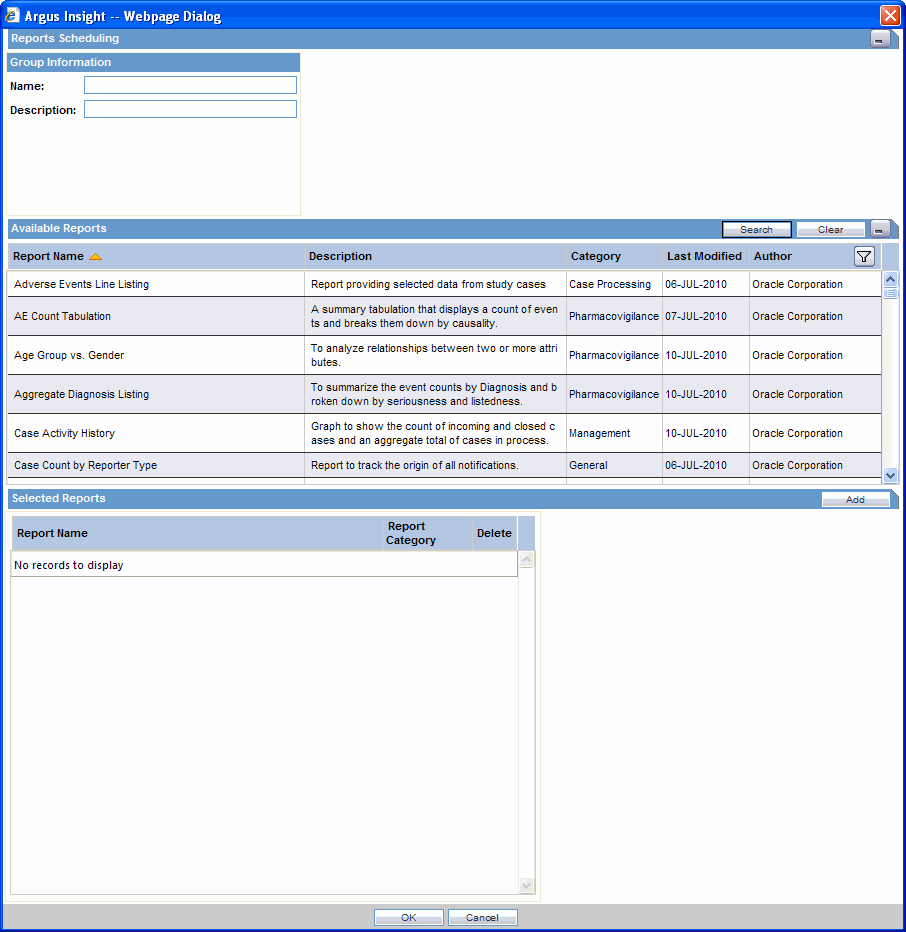
This the main scheduling page in Insight. On this page, user can select any number of reports for scheduling.
The Reports Scheduling page has four (4) sections as follows:
Group Information: The user provides the group name and description.
Schedule Information: The user provides the scheduling frequency.
Email Information: The user provides e-mail information for the receivers.
Available Reports: The user selects the reports to schedule. Select any report and click Add to add the report to Selected reports. The user can select multiple reports and add them at the same time.
Selected Reports: Reports associate with the query (as per the selection by the user from Available Reports section) are listed in this field.
User can remove a selected report by clicking the X button against report's row in the grid from the Selected Reports section.
Sorting and Search functionality is available for all the columns in the Available Reports Section.
Pagination is also available on the Available Reports Section.
The system does not permit the user to save a schedule without entering the pre-filter information for each report. The system uses the value in the Pre-Filters Configured? Column in the Selected Reports section to detect whether the pre-filter configuration is complete.
The user can enter pre-filter information by selecting any report in the Selected Reports section. When the user selects the report, the system loads the pre-filter information for the report in the sidebar and enables the user to enter the information.
Side bar has OK and Cancel buttons
OK: Save the pre-filter information for the selected report.
Clear: Reset the pre-filter page to blank values after confirming with user.
The Schedule Information page has the following buttons:
OK: Saves the scheduling information to the database and returns to the Scheduled Report Groups page.
Cancel: Returns to the Scheduled Report Groups page without making any changes.
Clear: Resets the Schedule Information page to blank values after confirming with user
Reports can be scheduled without a query from the Associated Library Page.
This page has a button named "Associate to <All Cases>."
The user can configure Text All Cases can from the List Maintenance items.
The system appends configured text to "Associate to <LM Text>". For example, if we configure All System Cases in the LM item, the text of this button would be "Associate to All System Cases".
The default value is All Cases.
The name of the LM Item is "All Cases Query Name".
When the user clicks this button, the system opens the Scheduled Report Groups window and behaves exactly the same way as described in the section, "Multiple Reports on a Single Query".
The Associated Reports Library page includes the following features:
This page is the main status page for all scheduled reports.
The user can see all reports in different/same groups at the same time. For example, if a user has two (2) report groups with three (3) reports in each group, the library has six (6) rows, one for each report association.
The Status column displays the current status of the reports association.
This page has the following buttons:
Modify: Enables the user to modify a selected association. This opens the Scheduled Report Groups page to query of the selected association. The system highlights the current group of the selected association.
Execute: Executes the selected query to get the case series and opens the Pre-Filter page for the associated report.
The Standard Reports page displays a list of all the Standard Reports built into Argus Insight. Select Case Series Reports > Standard Reports > All to display the Standard Reports page. This page displays a list of the saved Standard Reports in a grid format. You can search for specific types of reports by specifying values for any of the columns as the search criteria and clicking Search.
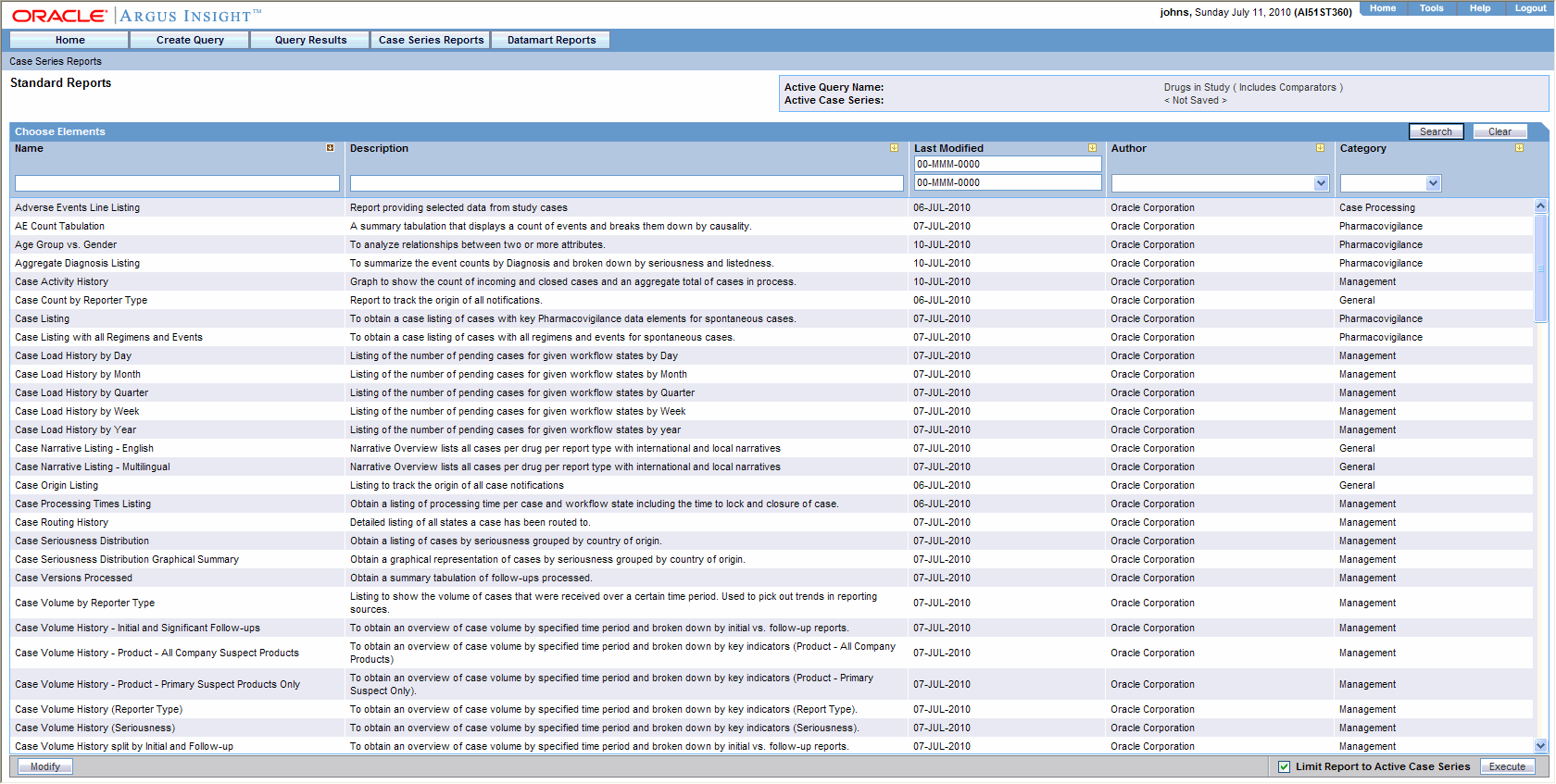
The descriptions of the grid columns follow.
| Column | Description |
| Name | Displays the name of the Standard Report |
| Description | Displays the Standard Report description |
| Last Modified | Displays the date when the Standard Report was last modified |
| Author | Displays the name of the Standard Report author |
| Category | Displays the Standard Report Category |
Standard Reports are grouped into six categories and can be accessed from the Argus Insight folder in the Installation CD. The following topics explain the various standard reports.
Compliance Reports
Configuration Reports
General Reports
Management Reports
Pharmacovigilance Reports
The following image displays the Cover page of the report when it is generated in PDF format.
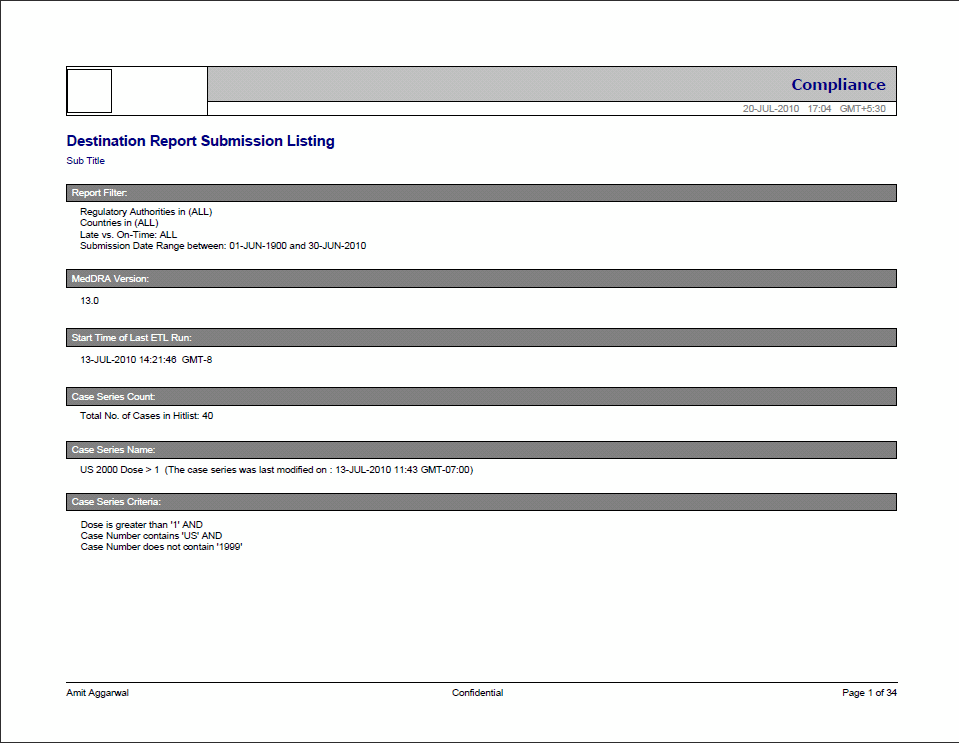
The following table describes each component of the cover page shown in the above image.
| Cover Page Component | Description |
| Company logo (upper-left box) | Placeholder for a configurable logo. The image of the logo can be configured in the whitespace. |
| Report Category (upper-right box) | The name of the category to which the selected report belongs. |
| Report Execution Date (under 'Report Category') | Report Execution Time (in GMT). |
| Report Title (under the Heading of the Report) | Title of the selected report, such as Serious Adverse Events Report. |
| Report Sub-Title (under 'Report Title') | Configurable Sub-title of the selected report. You can enter a sub-title to further define the report. Example: 01-JAN-1900 to 31-DEC-2007. |
| Report Filter | A selected value from the Report Prompt. |
| MedDRA Version | The MedDRA Version for the selected report. |
| Start Time of Last ETL Run | Start time of the last successful ETL run (in GMT). |
| Case Series Count | A count of the total number of unique cases in the case series. |
| Case Series Name | The name of the saved case series on which the report has been executed. Reports that are run on frozen case series data indicate on the report next to the case series name that "The data in this report was frozen as of <particular date and time>". Time is the database system time with the GMT offset.Reports that are run on normal case series data indicate on the report next to the case series name that "The case series was last modified on <Case series modification date and time>". |
| Case Series Criteria | Query criteria to get case series on which the report has been executed. |
| User Name (bottom-left) | The name of the user who executed the report. |
| Confidential (bottom-centre) | Configurable Confidential text. |
| Page x of y (bottom-right) | Page Number (in Current Page number of Total Pages in the Report format). |
| Footer logo (bottom-right box) | Placeholder for a configurable logo. The image of the logo can be configured in the whitespace. This component may not appear in every report. |
The following table describes each Compliance Report.
| Report Title | Description |
|---|---|
| Destination Report Submission Listing | Use this report to view the submission details based on an on-line country. |
| Global Audit Listing | Use this report to view case information relating to adverse event.
This report is a listing of case information relating to adverse event, listedness, seriousness, and submission record information for suspect products, used to support external audit. |
| KPI List Report Country Group 1 | Listing of case submission information for submissions in Group 1 countries (countries with clock starting from first receipt globally) grouped by Agency (submission destination) to support compliance KPI generation. |
| KPI List Report Country Group 2 | Listing of case submission information for submissions in Group 2 countries (countries with clock starting from receipt in that country, i.e aware date for domestic cases and generated date for foreign cases) grouped by Agency(submission destination) to support compliance KPI generation. |
| KPI Overview Report Group 1 | Table of submission count, late submission count and overall compliance figures for submissions in Group 1 countries (countries with clock starting from receipt globally) grouped by Agency(submission destination) to support compliance KPI generation. |
| KPI Overview Report Group 2 | Table of submission count, late submission count and overall compliance figures for submissions in Group 2 countries (countries with clock starting from receipt in that country, i.e aware date for domestic cases and generated date for foreign cases y) grouped by Agency(submission destination) to support compliance KPI generation. |
| Labeling Detail Listing by Case Number | Use this report to check for consistency between Labeling and Seriousness Criteria. The report is a listing of cases with events and their labeledness for the licenses of selected countries. Only the first company suspect product is included in the report; however, all events are listed. This report is grouped by case numbers. |
| Labeling Detail Listing by Preferred Term | Use this report to check for consistency between Labeling and Seriousness Criteria. This report is a listing of cases with events and their labeledness for the licenses of the selected countries selected. Only the first company suspect product is included in the report; however, all events are listed This report is grouped by Preferred Term. |
| Labeling Summary | Use this report to verify the consistency of labeling assessment. This report is a summary tabulation of labeling for events based on the countries selected. |
| Outstanding Expedited Reports by Responsible Group | Use this report to view a listing of cases for which expedited reports are outstanding. The listing is grouped by the responsible group. |
| Outstanding Report Submission by Responsible Group Listing - Detail | Use this report to view a detailed listing of cases for which report submissions are coming up.
The listing is grouped by responsible group and again sub-grouped by agency. The listing is sorted in descending order of due-date. |
| Outstanding Report Submission by Responsible Group Listing - Summary | Use this report to view the outstanding report count for each reporting destination. Against each destination, the outstanding reported count is further broken up in these groups:
|
| Outstanding Report Submissions by Destination Listing - Detail | Use this report to obtain a detailed listing of upcoming report submissions based on report destinations. |
| Outstanding Report Submissions by Destination Listing - Summary | Use this report to obtain a summary listing of upcoming report submissions based on report destinations (Summary). |
| Reporting Compliance by Destination (Count) | Use this report to obtain a graphical overview of reporting compliance. |
| Reporting Compliance by Destination (Percentage) | Use this report to obtain a graphical overview of compliance expressed in percentages. |
| Reporting Compliance by Grouped Destination (Count) | Use this report to obtain a graphical overview of compliance grouped by reporting destination. |
| Reporting Compliance by Grouped Destination (Percentage) | Use this report to obtain a graphical overview of compliance grouped by reporting destination expressed in percentages. |
| Reporting compliance by Responsible Group | Use this report to obtain a graphical overview of compliance for a given reporting group. This report shows how many reports were assigned to a particular reporting group, that were submitted before the due date/ on the due date/ after the due date. |
| Reporting Compliance Listing - Detail | Use this report to view a detailed listing of submitted as well as outstanding reports for the selected cases and reporting destinations. The listing is grouped by destination; subtotals and compliance % are provided for each destination along with an overall total and total compliance %. |
| Reporting Compliance Listing - Summary | Use this report to view a summary listing of submitted as well as outstanding reports for the selected cases and reporting destinations. The listing is grouped by destination; subtotals are provided for each destination along with compliance percentage. |
| Reports Out of Compliance Listing - Detail | Use this report to view a detailed listing of all reports that were past the due date for the selected cases and reporting destinations. The listing is grouped by reporting destination; subtotals are provided for each destination apart from the overall total. |
| Reports Out of Compliance Listing - Summary | Use this report to view a summary listing of all reports that were past the due date for specific destinations. The listing is grouped by reporting destination; subtotals are provided for each destination apart from the overall total. |
| Reports Out of Compliance with Delayed Workflow Detail | Use this report to view a listing of late reports along with the details of the workflow states where they exceeded the maximum allotted time. |
The following table describes each Configuration Report.
| Report Title | Description |
| ETL Log Argus to Staging Incremental | Displays the ETL Log for the latest Argus to Staging ETL Incremental Process. |
| ETL Log Staging to Mart Incremental | Displays the ETL Log for the latest Staging to Data Mart ETL Incremental Process. |
| ETL Log Summary | Displays the ETL Summary for all the ETL Processes. |
| Listing of Licenses by Family and Product | Obtain an overview of defined licenses grouped by families. |
| Listing of Product by Family and Licenses | Obtain an overview of defined products grouped by families. |
| StudyConfiguration | Obtain a listing of configured Studies. |
| Workflow Configuration by State | Obtain a listing grouping the workflow states listing the incoming and outgoing states. |
| Workflow Configuration by Transition | Obtain a listing of the configured transitions sorted by From and Use the following procedure to states. |
| Reporting Rules Configuration | Obtain a listing of configured Reporting Rules. |
The following table describes each General Report.
| Report Title | Description |
|---|---|
| Audit Review Listing | This report provides a listing of case information in tabular format relating to adverse event, listedness, seriousness for Novartis suspect products, used to support internal audit. |
| Case Count By Reporter Type | This is a report for tracking the origin of all notifications. |
| Case Narrative Listing -English | This report is a narrative overview list of all cases per drug per report type with international and local narratives |
| Case Narrative Listing -Multilingual | This report is a narrative overview list of all the cases per drug per report type with international and local narratives |
| Case Origin Listing | This report is a listing to track the origin of all case notifications. |
| CIOMS II Line Listing | This report prints the standard CIOMS II Listing report for all cases of the case series. |
| CIOMS Report | This report prints the standard CIOMS I report for all cases of the case series. |
| Clinical Medical Review List | This report provides a listing of serious clinical trial events grouped by study ID. |
| Detailed Line Listing by Case Number | This report is a listing of case details (suspect drugs, concom drugs, events, outcome, indication, narrative, demographics, relevant history) grouped by study number, center number, patient number and sorted by case number. Used to support data reconciliation process. |
| Detailed Line Listing by SOC | This report is a listing of case details (suspect drugs, concom drugs, events, outcome, indication, narrative, demographics, relevant history) grouped by SOC and sorted by SOC to support medical review, analysis and documentation. This listing is used widely and often provided to internal and external customers. |
| Downgraded Reports Listing | This report obtains a listing of downgraded reports. |
| Literature Listing | This report provides an overview including case number with literature reference and case details. |
| Lot Number Listing - Detail | Use the following procedure to investigate a correlation of events for a certain lot number (Listing). |
| Lot Number Listing - Summary | Use the following procedure to investigate a correlation of events for a certain lot number (Summary). |
| MSE Review List For Non Serious SR | This report lists the basic case information(events, suspect drugs) - grouped by MSE (based on MSE-Drug responsibility configuration) to assist MSE in weekly review of non-serious SR and unrelated CT cases |
| MSE Review List for Unrelated CT | This report lists the basic case information(events, suspect drugs) - grouped by MSE (based on MSE-Drug responsibility configuration) to assist MSE in weekly review of non-serious SR and unrelated CT cases |
| Relevant Medical History listing | This report is a listing of all relevant histories for the selected cases. |
| SAE Clinical Trial Co-Medication | This report is a listing of case concomitant medication details - sorted by case number. Used to assist medical investigation. |
| SAE Clinical Trial Detail Listing | This report is a listing of case details(events, demographics, study details, treatment details) - sorted by case number. Used to assist medical investigation. |
| SAE Clinical Trial Laboratory Test | This report is a listing of case laboratory tests - sorted by case number. Used to assist medical investigation |
| SAE Clinical Trial Medical Conditions | This report is a listing of case current conditions, family history, past medical history - sorted by case number. Used to assist medical investigation. |
| SAE Clinical Trial Narratives | This report is a listing of case narratives and study details - grouped by protocol number and sorted by case number. Used to assist medical investigation. |
| SAE Clinical Trial Project Overview | This report is in tabular data of number of distinct cases and receipt date range, create date range flags - grouped by protocol number, study number, report type. |
| Simple Line Listing by Case Number | This report is a listing of key case information - grouped and sorted by case number to provide high level overview and quick review. |
| Simple Line Listing by SOC | This report is a listing of key case information - grouped and sorted by SOC of primary event, drug, and case number to provide high level overview and quick review. |
| Study Reconciliation Report | This report provides a data-set to enable reconciliation between Argus and the company clinical database. |
| US FDA MedWatch 3500A | Prints the standard US FDA MedWatch 3500A report for all cases of the case series. |
The following table describes each Management Report.
| Report Title | Description |
|---|---|
| Case Load History by Day | Listing of the number of pending cases for given workflow states by Day |
| Case Load History by Month | Listing of the number of pending cases for given workflow states by Month |
| Case Load History by Quarter | Listing of the number of pending cases for given workflow states by Quarter |
| Case Load History by Week | Listing of the number of pending cases for given workflow states by Week |
| Case Load History By Year | Listing of the number of pending cases for given workflow states by year |
| Case Processing Times Listing | Obtain a listing of processing time per case and workflow state including the time to lock and closure of case. |
| Case Routing History | Detailed listing of all states a case has been routed to. |
| Case Seriousness Distribution | Obtain a listing of cases by seriousness grouped by country of origin. |
| Case Seriousness Distribution Graphical Summary | Obtain a graphical representation of cases by seriousness grouped by country of origin. |
| Case Versions Processed | Obtain a summary tabulation of follow-ups processed. |
| Case Volume by Reporter Type | Listing to show the volume of cases that were received over a certain time period. Used to pick out trends in reporting sources. |
| Case Volume History - Initial and Significant Follow-ups | Use the following procedure to obtain an overview of case volume by specified time period and broken down by initial vs. follow-up reports. |
| Case Volume History - Product - All Company Suspect Products | Use the following procedure to obtain an overview of case volume by specified time period and broken down by key indicators (Product - All Company Products) |
| Case Volume History - Product - Primary Suspect Products Only | Use the following procedure to obtain an overview of case volume by specified time period and broken down by key indicators (Product - Primary Suspect Only). |
| Case Volume History (Reporter Type) | Use the following procedure to obtain an overview of case volume by specified time period and broken down by key indicators (Report Type). |
| Case Volume History (Seriousness) | Use the following procedure to obtain an overview of case volume by specified time period and broken down by key indicators (Seriousness). |
| Case Volume History split by Initial and Follow-up | Use the following procedure to obtain an overview of case volume by specified time period and broken down by initial vs. follow-up reports. |
| Case-Load | Obtain a graphical representation of cases in each workflow state. |
| Case-Load Listing | Obtain a listing of the number of cases received and processed per workflow state for a given time period. |
| Cases by Reporter Type Tabulation | Use the following procedure to compare the number of cases from each reporter type. |
| Delayed Workflow Listing by Case | Obtain a list of cases where the max time has been exceeded. |
| Delayed Workflow Listing by Workflow State | Obtain a list of cases where the max time has been exceeded. |
| First and Last Reporter Contact | Obtain a listing of case details including first and last contact from the reporter (Initial receipt and most recent follow-up date) for each case. |
| Follow-up Status Listing | Obtain a listing of cases requiring follow-up and the status of those cases. |
| Listing of Cases due for Lock | Obtain a listing of cases that due for lock |
| Listing of Cases Late for Lock | Obtain a listing that displays case work state milestones and lead times for workflow as well as minimal case information. |
| Open Action Items Listing | Obtain a list of cases with open action items by responsible group. |
| Product Tabulation by Site and Case Source | Obtain a summary that lists the number of cases by site and product (Ingredient) by Report Type with totals. |
| Receipt Latency of Cases by Site - Initial and All Follow-ups | Obtain a listing of latency from initial receipt date to central received date for initial and follow-up reports. |
| Receipt Latency of Cases by Site - Initial and Significant Follow-ups | Obtain a listing of latency from initial receipt date to central received date for initial and follow-up reports. |
| Receipt Latency of Cases by Site and Country of Origin | Obtain a listing of latency from initial receipt date to central received date for initial and follow-ups, broken down by country of origin. |
| Received AE Reports by Protocol | Obtain a line Listing of all cases received grouped by Protocol Number (Study ID). |
| Report Volume History | Listing to show the volume of reports that were submitted over a certain time period broken down by reporting destination. |
| Reports By Causality | Graph of number of reports processed grouped by case causality to verify workload, productivity. The counts show the number of case locks (initial or follow-up) with initial or follow-up information during the report period. |
| Reports by Country | Graph of number of reports processed - grouped by country of incidence and sorted by descending counts to verify workload, productivity. The counts show the number of case locks (initial or follow-up) for cases originating from that country during the report period. |
| Reports by Month | Graph of number of reports processed - grouped by month and sorted by numeric month to verify workload, productivity. The counts show the number of case locks (initial or follow up) in that month. |
| Reports By Product Family | Graph of number of reports processed - grouped and sorted by descending counts to verify workload, productivity. The counts show the number of case locks (initial or follow up) where the primary suspect is in that Drug Family during the report period. |
| Reports by Report Type | Graph of number of reports processed - grouped by report type; such as Spontaneous Report, Clinical Trial(non-PMS), PMS, or Literature. The counts show the number of case locks (initial or follow-up) during the report period. |
| Reports by Seriousness | Graph of number of reports processed - grouped by case seriousness to verify workload, productivity. The counts show the number of case locks (initial or follow-up) during the report period. |
| Reports by Year | Graph of number of reports processed - grouped by year and sorted chronologically. The counts show the number of case locks (initial or follow-up) in that year. |
| Total Case WorkLoad by Site | Obtain a listing of total number of cases in the system. |
| Total Case Workload by Site and COI - Init. and Sig. Follow-ups | Obtain a listing of total number of cases in the system grouped by site and country of origin. |
| Total Case Workload by Site and COI - Initial and All Follow-ups | Obtain a listing of total number of cases in the system grouped by site and country of origin. |
| Total Case Workload by Site Graphical Summary | Obtain a graphical overview of the total number of cases in the system broken down by seriousness. |
| Workflow Monitoring Report | Obtain a listing that displays case workflow milestones and lead times for workflow as well as minimal case information. |
| Workflow Report - 3 months | Counts based on Groups over 3 month periods, based on a selected date range. |
| Workflow Report - 4 quarters | Counts based on Groups over 4 quarters, based on a selected date range. |
The following table describes each Pharmacovigilance Report.
| Report Title | Description |
|---|---|
| AE Count Tabulation | This report is a summary tabulation that displays a count of events and breaks them down by causality. |
| Age Group Vs. Gender | This report is an analysis of relationships between two or more attributes. |
| Aggregate Diagnosis Listing | This report is a summary of the event counts by Diagnosis and broken down by seriousness and listedness. |
| Annual Product Review | This report is a listing of all cases for a drug family grouped by primary suspect drug, SOC and sorted by drug, SOC to review cases potentially associated with quality compliant or lack of efficacy. |
| Case Listing | This report is a case listing of cases with key Pharmacovigilance data elements for spontaneous cases. |
| Case Listing with all Regimens and Events | This report is a case listing of cases with all regimens and events for spontaneous cases. |
| Clinical Case Listing | This report is a case listing for clinical cases |
| Clinical Trial Causality | This report captures the Table of event count grouped by selected MedDRA levels (SOC,HLGT,HLT,PT), administered drug, event causality to support medical investigation. Case counts for selected MedDRA levels and administered drug is also included |
| Clinical Trial Causality Tabulation | This report is required to tabulate the causality assessments for each occurring PT. |
| Count of Serious Related Cases by Product | This report provides an overview of serious related cases over a given time period. |
| Data Quality Indicator (Alphabetically by Quality Indicator) | This report provides a graphical summary of case data quality for the following key data elements |
| Data Quality Indicator Listing | This report provides a list that contains the cases that contributed to the data value as the graphical report |
| Data Quality Indicator (Ascending Order of Count) | This report provides a graphical summary of case data quality for the following key data elements. |
| Death Cases | This report provides the listing of cases with death details, event details where the patient has had a fatal outcome - grouped by SOC. |
| Dechallenge and Rechallenge Listing | This report provides the Line Listing for Dechallenge and rechallenge information |
| Dosage Frequency Tabulation | This report captures the frequency of dosing for a particular product to see if there is an increase in frequency of various AEs. |
| Dose Formulation Product Relationship | This report captures the relationship between dosage formulation and events for a given suspect drug. |
| Duration of Treatment until Event (Time to onset) per Event | This report captures the table of event counts categorized by time to onset - grouped and sorted by event. |
| Event (PT) Vs Age Group | This report is required to analyze relationships between two or more attributes. |
| Event (PT) Vs Daily Dose | This report is required to analyze relationships between two or more attributes. |
| Event (PT) Vs Duration of Treatment Until Event | This report is required to analyze relationships between two or more attributes. |
| Event (PT) Vs Gender | This report is required to analyze relationships between two or more attributes. |
| Event (PT) Vs Report Type | This report is required to analyze relationships between two or more attributes. |
| Event Term Frequency Listing by HLGT | This report is required o identify the frequency of events in descending order, grouped by SOC, HLGT, HLT, or PT. |
| Event Term Frequency Listing by HLT | This report is required to identify the frequency of events in descending order, grouped by SOC, HLGT, HLT, or PT. |
| Event Term Frequency Listing by PT | This report is required to identify the frequency of events in descending order, grouped by SOC, HLGT, HLT, or PT. |
| Event Term Frequency Listing by SOC | This report is required to identify the frequency of events in descending order, grouped by SOC, HLGT, HLT, or PT. |
| Event Vs Daily Dose | This report is required to analyze relationships between two or more attributes. |
| Event Vs Report Type | This report is required to analyze relationships between two or more attributes. |
| Event Vs. Age Group | This report is required to analyze relationships between two or more attributes. |
| Event Vs. Duration of Treatment Until Event | This report is required to analyze relationships between two or more attributes. |
| Event Vs. Gender | This report is required to analyze relationships between two or more attributes. |
| Fatal Case Listing | This report is required to obtain a listing of cases with fatal seriousness criteria. |
| Fatal/Life-Threatening Cases Listing | This report is required to obtain a listing of cases with fatal or life-threatening seriousness criteria. |
| Frequency Listing of Events | This report is a table of event counts - sorted by descending event count frequency to help support labeling or package insert review. |
| Gender Group by Age Group | This report is required to capture the Gender Group by Age Group |
| MedDRA SOC-PT group report type, event seriousness, causality | This report is required to capture the Table of event, case count categorized by report type, event seriousness, causality - grouped and sorted by preferred term to support medical analysis. |
| MedDRA SOC-PT Group by Age Group | This report is required to capture the Table of event, case count categorized by chronological Age group - grouped by preferred term to support medical analysis. |
| MedDRA SOC-PT Group by Daily Dose | This report is required to capture the Table of event, case count categorized by Daily Dose bands - grouped and sorted by preferred term to support medical analysis |
| MedDRA SOC-PT Group by Gender | This report is required to capture the Table of event, case count categorized by gender - grouped and sorted by preferred term to support medical analysis. |
| Product and Disease Listing | This report captures the concomitant medications and underlying disease |
| Product Interaction Tabulation | This report is a summary tabulation that displays a count of the incidence of other products involved in cases. |
| Quick Signal | This report captures the event-reporting rates that might suggest a possible change in the safety profile of a product. |
| Seriousness Case Listing | This report provides a listing of cases by grouped by seriousness criteria. |
| SOC/PT Tabulation (Event Count) | This report compares events based on report type. |
| Temporal Relationships | This report captures a graphical overview of the temporal relationships of the dates recorded in the case. |
| Top 10 Substances | This report captures a listing of the top 10 frequent substances occurring in cases for the specified time period. |