| Oracle® Health Sciences Adverse Event Integration Pack for Oracle Health Sciences InForm and Oracle Argus Safety Implementation Guide Release 1.0.1 E36157-02 |
|
|
PDF · Mobi · ePub |
| Oracle® Health Sciences Adverse Event Integration Pack for Oracle Health Sciences InForm and Oracle Argus Safety Implementation Guide Release 1.0.1 E36157-02 |
|
|
PDF · Mobi · ePub |
This chapter provides an overview of the Adverse Event: InForm and Argus Safety Integration. This chapter includes:
Adverse Event: InForm and Argus Safety integration automates the process of clinical study sites reporting serious or clinically significant adverse events for a drug or a medical device from Oracle Health Sciences InForm to the Oracle Argus Safety system. This automation increases productivity by reducing the amount of reconciliation needed between the two systems. For instance, if an event is recorded in the Electronic Data Capture (EDC) system, the clock starts ticking for these reporting deadlines. This integration automates the sending of the information to Argus Safety so that the Safety users can start work on the case.
You can define which data should be sent to safety using Central Designer Logical Schemas. You can also define which data should trigger a follow-up to safety if the data is changed. Potentially related adverse events, labs, and concomitant medications are sent to safety based on time frames you configure.
If desired, the Argus Case #, whether the safety user accepted or rejected the E2B file, and the rejection reason can be sent back to InForm.
These are the key benefits of this integration:
Improves timeliness and completeness of Safety Serious Adverse Event (SAE) data
Increases productivity by eliminating duplicate data entry between Oracle Health Sciences InForm and Oracle Argus Safety
Reduces the need for reconciliation
Automates the sending of follow-up when significant data is changed
This section provides an overview of the participating applications.
Oracle Argus Safety is an advanced and comprehensive adverse events management system that helps life sciences companies enable regulatory compliance, drive product stewardship, and integrate safety and risk management into one comprehensive platform. Oracle Argus Safety is industry-proven as having been used for more than a decade at leading pharmaceutical, biotech, CRO (Clinical Research organization), and medical device manufacturers. It also facilitates internal company safety surveillance by analyzing the overall safety profile of both investigational compounds and marketed products.
Oracle's proactive approach to monitoring global guidance enables consistent regulatory compliance. Oracle Argus Safety supports electronic communication with trading partners and CROs, providing visibility into compliance across a company's global licensing partnerships.
Oracle Argus Safety also supports a company's end-to-end pharmacovigilance program by providing a simple and efficient way to comply with international and domestic regulatory safety reporting requirements from clinical trials through post-marketing surveillance. By integrating serious adverse events from clinical trials, companies have the opportunity to manage efficacy earlier in the drug lifecycle and can potentially address public health concerns sooner. It provides the most comprehensive global adverse events case data management and regulatory reporting in the pharmaceutical industry.
Oracle Health Sciences InForm Global Trial Management (InForm GTM) is an electronic data capture (EDC) system used by pharmaceutical and medical device companies to collect data during medical studies. Oracle Health Sciences InForm helps clinical research coordinators, trial monitors, clinical data managers, and project managers work more efficiently and with greater accuracy throughout the clinical development process. Its intuitive user interface, multilanguage capabilities, and associated software modules deliver a robust solution to meet the needs of today's global EDC users.
In addition, Oracle Health Sciences InForm GTM's scalable platform enables flexible, speedy study design and build, robust data management, and real-time data visibility, and provides user-driven reporting tools and advanced analysis capabilities. Further, interactive voice response systems, clinical trial management systems, imaging, and electronic patient reported outcomes can easily integrate with Oracle's e-clinical environment through the support of Web services and industry standards.
One type of information collected is Adverse Events (AE) that includes illnesses, pain, and other negative bodily effects. The AE events are documented in the Oracle Health Sciences InForm system, along with information about the event, such as medications taken, and current status of the AE. When the AE causes hospitalization or death, it is termed as a Serious Adverse Event (SAE) and safety reports are prepared for the regulatory authorities of studies. Other events that are determined to be of significant medical interest are also reported to the regulatory authorities of studies.
The Oracle Health Sciences InForm Adapter software provides interfaces to Web services that support the secure transfer of data between InForm studies and either Oracle products (such as the CIS and Central Coding applications) or third-party products and custom applications. Each application that can accept queries or updates to its data and metadata from InForm studies requires a specific set of interfaces.
Like application programming interfaces (APIs), the Oracle Health Sciences InForm Adapter interfaces use published Web services interfaces to allow programmatic access to applications. This lets Oracle products to be tightly integrated with each other and with third-party products.
For InForm-Argus integration, both Oracle Health Sciences InForm Publisher and the integration layer invoke InForm Adapter's Safety Web service to update safety event status information.
The Oracle Health Sciences InForm Publisher software publishes data from InForm studies to web service endpoints where it can be imported to a target application.
The data is published in real time from a transaction queue, or on a configurable schedule.
To use the Oracle Health Sciences InForm Publisher software with an InForm study, you must:
Install the Oracle Health Sciences InForm Publisher software on the InForm application server.
Configure the software to work with the study and the target application.
The following are the business process flows in the integration:
Reporting potential new cases to safety system
Sending follow-up for existing cases to safety
Sending nullification of an existing case to safety
Sending case ID and status from safety to Oracle Health Sciences InForm
Refer to Figure 1-1 and Figure 1-2 and the process description given below:
Figure 1-1 Business Process Model Part – 1
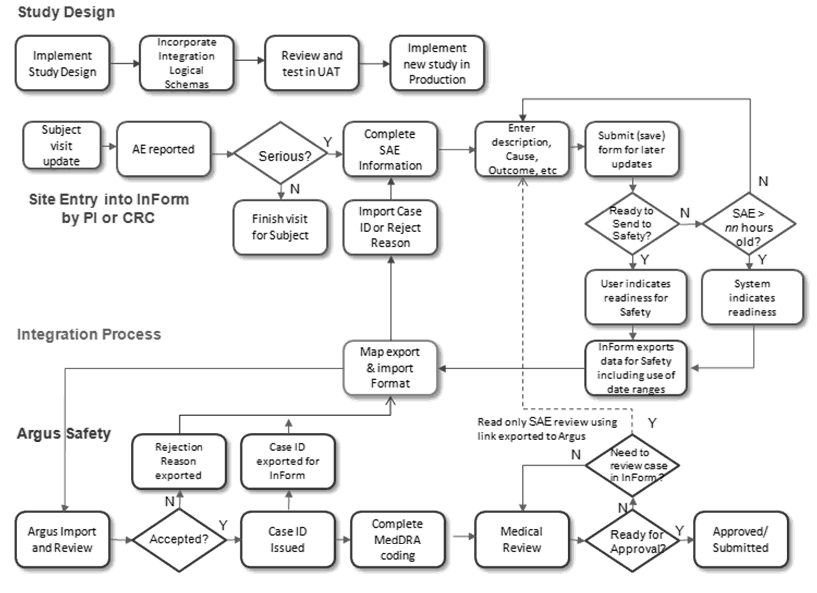
During the study design, the designer will map the items that need to be sent to the Safety Logical Schema and identify which items should trigger a follow-up. The trial will go through User Acceptance Testing (UAT) and then be implemented in production.
During a subject visit, AEs may be reported. If they are serious, the appropriate information will be entered and saved. Once the information is considered complete and ready to send to safety, the InForm user will select the check box. When the form is submitted the data will be exported and sent to the integration layer. The integration layer transforms the message to E2B+ format and performs any necessary transformations. A file will be written to the Argus Interchange server where it is imported to the Pending E2B screen.
The Argus user can accept the file as a new case or reject the file. If the file is accepted, the case number is returned to InForm. If the file is rejected, the reason for rejection is returned to InForm.
Figure 1-2 Business Process Model Part – 2
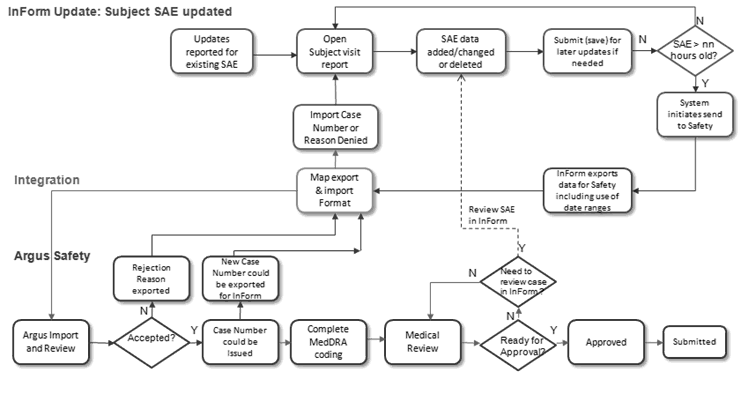
SAE data may be updated in InForm. You can configure the interval to check for follow-up. At that interval, the system sends the follow-up data to the integration layer if any significant data has changed. The integration layer transforms it to an E2B+ file and the file will be written to the Argus Interchange server.
The Argus user can choose to accept or reject the file. If the user accepts the follow-up into either an existing case or a new case, the case number is sent back to InForm. If the safety user rejects the file, the reason for rejection is sent back to InForm.
Figure 1-3 is the logical mapping between the high level entities in Oracle Health Sciences InForm and Oracle Argus Safety.
Figure 1-3 Logical Model Mapping Oracle Health Sciences InForm and Oracle Argus Safety
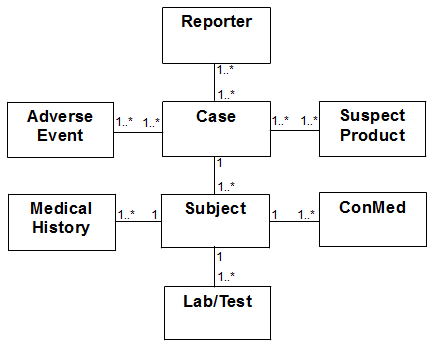
Each case has only one subject. There may be many cases for a given subject. Multiple related Adverse Events can be part of the same case. A case in Argus can have multiple reporters but one will be considered the primary reporter. A case can involve one or more suspect products.
Each subject has medical history, labs, and ConMeds.
Table 1-1 describes a high level mapping of the business objects in Oracle Health Sciences InForm, Oracle Argus Safety, DrugSafetyReport EBO, and E2B:
Table 1-1 Mapping of the Business Objects
| InForm | Argus | DrugSafetyReportEBO | E2B |
|---|---|---|---|
|
Serious Adverse Event |
Case |
DrugSafetyReport |
report |
|
User marking event as Serious |
Reporter (generally the principal investigator if coming from a study) |
DrugSafetyReportPrimarySource |
primarysource |
|
Subject |
Patient |
DrugSafetyReportPatient |
patient |
|
Prior Medications |
Patient Relevant History |
DrugSafetyReportPastDrugTherapy |
patientdrug |
|
Medical History |
Patient Relevant History |
DrugSafetyReportMedicalHistoryEpisode |
Patientepisode |
|
Adverse Event |
Event |
DrugSafetyReportReaction |
reaction |
|
Lab |
Lab Data Test |
DrugSafetyReportTest |
test |
|
Study Drug |
Suspect Product |
DrugSafetyReportDrug (characterization code:
|
drug characterization code:
|
|
Concomitant Medication |
Concomitant Medication |
DrugSafetyReportDrug(characterization code: 2) |
drug (characterization code: 2) |
|
Study Device |
Device Product |
DrugSafetyReportMedicalDevice |
drug |
|
Narrative text fields describing information relevant to the case. |
Case Narrative |
DrugSafetyReportSummary |
narrative |
Pending E2B reports in Oracle Argus Safety are processed in the order they are received. If it is not processed in the order they are received, the latest status and/or Safety System Case ID that is reflected in InForm can be inaccurate.
For example, if Oracle Health Sciences InForm sends an initial case and then a follow-up later that day, and the Oracle Argus Safety user accepts the follow-up as a new case in Oracle Argus Safety, and then views the initial case and rejects it because it does not display the latest information, in this scenario, the case status is shown as rejected in Oracle Health Sciences InForm.
Vaccine trials are not supported in this release. Vaccine information is not supported by Oracle Argus Safety E2B+ interface. This functionality will be considered for a future release once it is supported by Argus Safety.
Japanese version of Argus (Argus J) is not supported by the initial release of this integration.
The following study design is currently not supported:
Two or more separate forms to collect Adverse Events (AE). Currently, two or more AE forms are only supported if you are using a single form to collect AE and serious adverse events (SE).