| Oracle® Health Sciences Clinical Development Analytics User's Guide Release 3.2 E74647-01 |
|
|
PDF · Mobi · ePub |
| Oracle® Health Sciences Clinical Development Analytics User's Guide Release 3.2 E74647-01 |
|
|
PDF · Mobi · ePub |
This chapter contains the following topics:
Oracle Health Sciences Clinical Development Analytics (OHSCDA) is an analytical and transactional reporting application based on a predefined set of key performance indicators (KPIs), facts, and dimensions with support for predefined and custom reporting. OHSCDA provides rich support for merging data from multiple source databases into its single warehouse, providing a cross-enterprise view of operational data. OHSCDA also functions as a decision support system to monitor process bottlenecks and compliance deviations.
Clinical Development Organizations require insights into the following key Clinical Data Management and Clinical Trial Operations Management business processes areas that impact clinical trial performance:
Clinical Data Collection Management: All required data must be collected with no data omitted or wrongly entered using electronic data capture.
Clinical Data Cleanliness Management: Each discrepancy must be resolved, either by correcting the data that gave rise to it or by changing the rules that define the discrepancy.
Study Startup and Site Activation: All sites that are selected to recruit subjects and administer the study must be activated. Impediments to site activation must be identified and resolved.
Subject Recruitment: Study team must monitor if the subjects are enrolling, and the rate at which they are enrolling. If required, additional sites must be identified.
Site Monitoring Visits: All internal standard operating procedures must be met and all required data relevant to subject visits must be collected properly and on time.
OHSCDA provides mechanisms to gain operational and clinical insights to and evaluate performance of your clinical trials, and take corrective action to reduce cycle times.
OHSCDA integrates clinical trial performance data from multiple sources (Oracle Clinical, Oracle's Siebel Clinical, and InForm) into a predefined data warehouse schema and generates both predefined and custom reports of key metrics across the clinical development spectrum. OHSCDA lets you perform the following functions:
Extract all necessary clinical trial data from Oracle Clinical and Siebel Clinical into a predefined data warehouse, for viewing through the rich dashboard and report interface of Oracle Business Intelligence Enterprise Edition Plus (OBIEE).
Extract such data from other database sources as well, subject to the development of separate Extract Transform Load (ETL) programs for each database source.
Identify and merge duplicates while integrating data from multiple sources.
View predefined analytical reports delivered with the application.
Rapidly create new reports using the extensive predefined cycle time, quality and volume based metrics across data management and clinical operations processes.
Figure 1-1 displays the OHSCDA architecture.
Figure 1-1 Oracle Health Sciences Clinical Development Analytics Architecture
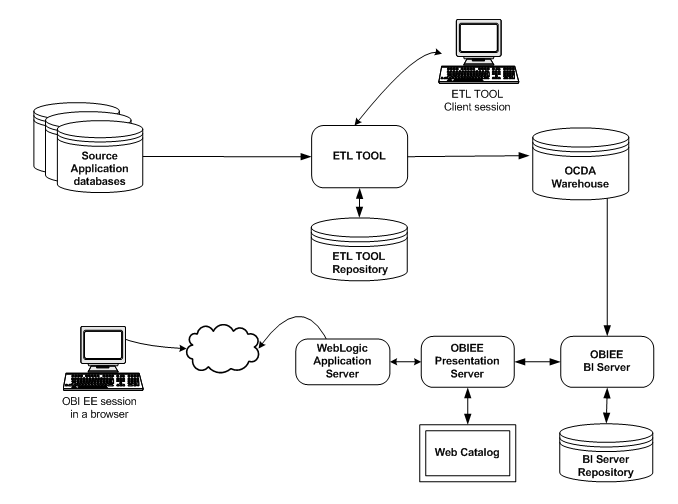
The OHSCDA architecture includes the following principal components:
A predefined Intelligence application, based on Oracle Business Intelligence Enterprise Edition Plus (OBIEE), including Oracle BI Presentation Services, and a predefined set of Oracle BI reports, accessible through prebuilt interactive dashboards.
A prebuilt clinical data warehouse schema, designed to drive both predefined and custom intelligence reports.
Oracle Data Integrator (ODI) based Extract Transform Load (ETL) programs, designed to extract clinical trial transactional data from Oracle Clinical and Siebel Clinical databases into the data warehouse schema.
A predefined BI Server Repository including a unique mix of clinical measures (facts, dimensions), populated by the data warehouse schema.
OHSCDA provides reports for the following key functional areas:
Clinical Data Collection Management: Reports on the data management workload and the rates at which work is progressing to provide insights to help minimize time to data lock.
Clinical Data Cleanliness Management: Cleanliness reports track the discrepancy-resolution workload, rates of progress, durations in various statuses, and provide drill-down detail.
Study Startup and Site Activation: Reports on sites that have not been activated and where they are in the process provide insight to help meet the recruitment goals.
Subject Recruitment: Reports on subject enrollment helps evaluate if additional sites are needed or if protocol amendment is necessary.
Site Monitoring Visits: Reports analyzing key metrics across the clinical monitoring process helps maintain compliance with internal standard operating procedures.
Risk Based Monitoring: Reports that track key risk indicators for both Study and Study-Site to help determine Study and Study-Site risk.
See Also:
OHSCDA is designed as a single data-warehousing platform that facilitates integration of both non-regulated and regulated data. This single platform provides secure access to authorized users. It provides reduced total cost of ownership through reduced data integration costs and infrastructure maintenance costs, compared with multiple warehousing solutions.
The primary regulatory requirements include: (i) data tracking and (ii) Extract Transform Load (ETL) Version Management.
The origin of any data displayed in a report must be traceable to its source, and all transformations applied to the data must be accessible. Data sourced from Oracle Clinical and Siebel Clinical is traced by the following criteria and rules:
Load: When the data was loaded from the source database into the staging tables.
Staging Mapping: The version of ETL mapping used to transform the data from source to staging table, and when it was executed.
Target Mapping: The version of ETL mapping used to transform the data from the staging table to target tables, and when it was executed.
Merged Dimensional Data: Records that were used to created the merged record.
Transformations and calculations performed on the data within the OBIEE Repository can be versioned and saved permanently in a third-party versioning tool.
Calculations can also be performed in reports managed through OBIEE Answers. The OBIEE Answers Administrator is responsible for controlling what calculations are performed, and who can perform them.
You can use a third-party versioning tool or in-built functionality of Informatica to manage ETL versions.
Data within the data warehouse is secure from updates by unauthorized personnel, and can only be updated through controlled execution of ETL mappings.
The ability to modify ETL routines is restricted to a user group or role of ETL developers. Access to execute ETL routines is restricted to a specific privilege, which can be granted to a user group or role.
In addition, access to data is available for authorized personnel only constrained through user groups or roles.
See Also:
Oracle Clinical Administrator's Guide