| Oracle® Argus Safety Flexible Aggregate Reporting Extensibility Guide Release 8.1 E75399-01 |
|
 Previous |
 Next |
| Oracle® Argus Safety Flexible Aggregate Reporting Extensibility Guide Release 8.1 E75399-01 |
|
 Previous |
 Next |
BI Publisher Periodic Reporting enables flexible handling of periodic reports in Argus Safety. The Oracle Argus Safety Flexible Aggregate Reporting (Oracle Argus FAR) Guide provides Argus Safety–BI Publisher integration details, and out-of-the-box periodic report details along with data models, algorithms, and methods to customize or extend these reports as needed.
This preface includes the following topics:
This product is part of the Oracle Health Sciences Safety Suite, an integrated solution for end-to-end vigilance from adverse event management to signal management, through the entire lifecycle of a medicinal product from clinical trials to post-marketing surveillance.
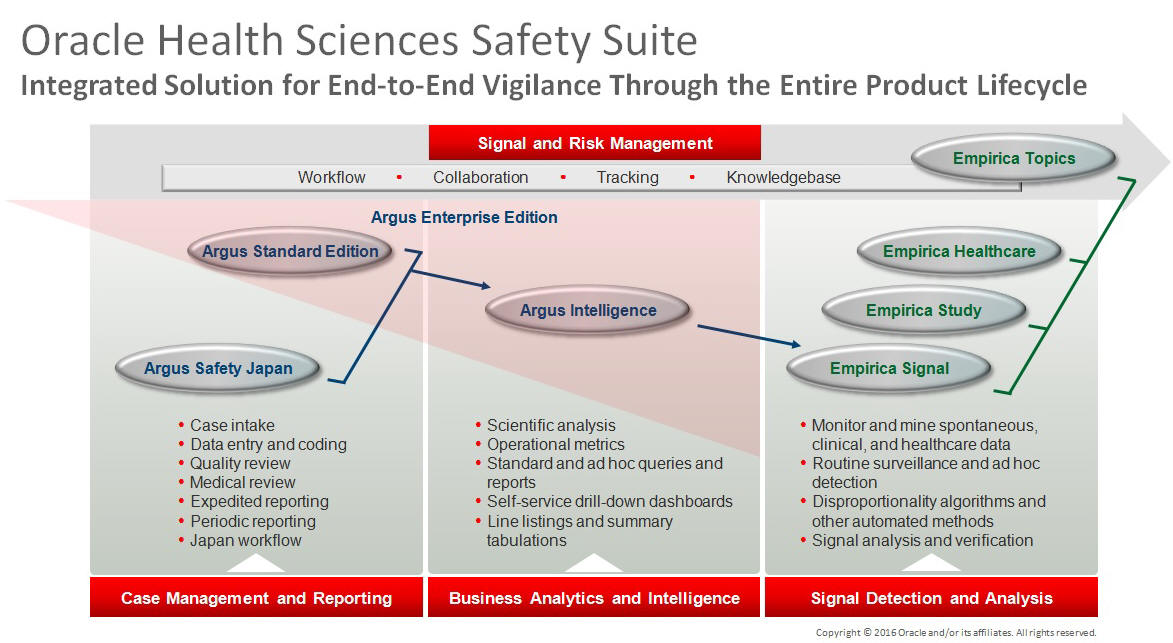
The Oracle Health Sciences Safety Suite consists of the following components:
Oracle Argus Standard Edition: Manage and report adverse events through a workflow including case intake, data entry, coding, quality review, medical review, expedited reporting, and periodic reporting. Modules include Oracle Argus Safety, Oracle Argus Interchange, Oracle Argus Affiliate, Oracle Argus Dossier, Oracle Argus Unblinding, and the Oracle Health Sciences Adverse Event Integration Pack for Oracle Health Sciences InForm and Oracle Argus.
Oracle Argus Enterprise Edition: In addition to managing the adverse event workflow and reporting, employ a powerful and flexible business analytics and intelligence platform for both scientific analysis and operational metrics. Modules include Oracle Argus Analytics, Oracle Argus Insight, Oracle Argus Mart, Oracle Argus Safety, Oracle Argus Interchange, Oracle Argus Affiliate, Oracle Argus Dossier, Oracle Argus Unblinding, and the Oracle Health Sciences Adverse Event Integration Pack for Oracle Health Sciences InForm and Oracle Argus.
Oracle Argus Safety Japan: Manage and report adverse events in Japan, and connect the global and local workflows using a single database.
Oracle Health Sciences Empirica Topics: Manage and document safety signals through a workflow including validation, prioritization, assessment, confirmation/refutation, and resulting actions.
Oracle Health Sciences Empirica Study: Detect and analyze safety signals in clinical trial data including adverse events, clinically significant labs, electrocardiograms, vital signs, and shifts from baseline.
Oracle Health Sciences Empirica Signal: Detect and analyze safety signals in post-marketing spontaneous adverse reaction data including public health authority databases and/or private inhouse databases such as Oracle Argus.
Oracle Health Sciences Empirica Healthcare Analysis: Evaluate safety signals in healthcare data including electronic medical records and administrative claims, and support pharmacoepidemiology, comparative effectiveness analysis, and health economics and outcomes research.
For more information on Argus Safety, visit the Oracle Health Sciences Safety suite page at:
http://www.oracle.com/goto/pharmacovigilance.html
This guide assumes that your organization has the expertise to perform the job functions listed in this section. If your staff lacks these skills, we recommend that you engage Oracle Health Sciences Consulting.
Customizing the database package supplied with Oracle Argus FAR requires a level of knowledge equivalent to having mastered the material in Oracle's DBA Architecture and Administration course. You must be able to read SQL*Plus scripts and edit them. You must be able to run SQL scripts and review logs for Oracle errors.
Customizing and maintaining an Oracle Argus Safety BI Periodic Reporting requires mastery of the following tools:
Microsoft Windows Operating System
Unix or Linux based Operating Systems
OBIEE and (or) Oracle BI Publisher
Oracle Web Logic Administration
For information about Oracle's commitment to accessibility, visit the Oracle Accessibility Program website at http://www.oracle.com/pls/topic/lookup?ctx=acc&id=docacc.
Access to Oracle Support
Oracle customers that have purchased support have access to electronic support through My Oracle Support. For information, visit http://www.oracle.com/pls/topic/lookup?ctx=acc&id=info or visit http://www.oracle.com/pls/topic/lookup?ctx=acc&id=trs if you are hearing impaired.
For more information, see the following documents in the Oracle Argus Safety documentation set:
Argus Safety Affiliate User Guide
Argus Safety Administrator's User Guide
Argus Safety Dossier User Guide
Argus Safety Interchange User Guide
Argus Safety Installation Guide
Argus Safety Service Administrator Guide
Argus Safety Flexible Aggregate Reporting Extensibility Guide
Argus Safety BIP Aggregate Reporting User's Guide
Argus Safety User's Guide
Argus Safety Unblinding User Guide
Argus Safety Minimum Security Configuration Guide
Argus Safety Japanese Administrator's Guide
Argus Interchange Japanese User's Guide
Argus Safety Japanese User's Guide
Your source for the latest information about Argus Safety is Oracle Support's self-service website My Oracle Support.
For more information on Argus Safety, visit the Oracle Health Sciences Safety suite page at:
http://www.oracle.com/goto/pharmacovigilance.html
Before you install and use Argus Safety, always visit the My Oracle Support website for the latest information, including alerts, White Papers, and bulletins.
You must register at My Oracle Support to obtain a user name and password account before you can enter the website.
To register for My Oracle Support:
Open a web browser to https://support.oracle.com.
Click the Register link to create a My Oracle Support account. The registration page opens.
Follow the instructions on the registration page.
To sign in to My Oracle Support:
Open a web browser to https://support.oracle.com.
Click Sign In.
Enter your user name and password.
Click Go to open the My Oracle Support home page.
There are many ways to find information on My Oracle Support.
The fastest way to search for information, including alerts, White Papers, and bulletins is by the article ID number, if you know it.
To search by article ID:
Sign in to My Oracle Support at https://support.oracle.com.
Locate the Search box in the upper right corner of the My Oracle Support page.
Click the sources icon to the left of the search box, and then select Article ID from the list.
Enter the article ID number in the text box.
Click the magnifying glass icon to the right of the search box (or press the Enter key) to execute your search.
The Knowledge page displays the results of your search. If the article is found, click the link to view the abstract, text, attachments, and related products.
You can use the following My Oracle Support tools to browse and search the knowledge base:
Product Focus — On the Knowledge page under Select Product, type part of the product name and the system immediately filters the product list by the letters you have typed. You do not need to type Oracle. Select the product you want from the filtered list and then use other search or browse tools to find the information you need.
Advanced Search — You can specify one or more search criteria, such as source, exact phrase, and related product, to find information. This option is available from the Advanced link on almost all pages.
Be sure to check My Oracle Support for the latest patches, if any, for your product. You can search for patches by patch ID or number, or by product or family.
To locate and download a patch:
Sign in to My Oracle Support at https://support.oracle.com.
Click the Patches & Updates tab. The Patches & Updates page opens and displays the Patch Search region. You have the following options:
In the Patch ID or Number field, enter the number of the patch you want. (This number is the same as the primary bug number fixed by the patch.) This option is useful if you already know the patch number.
To find a patch by product name, release, and platform, click the Product or Family link to enter one or more search criteria.
Click Search to execute your query. The Patch Search Results page opens.
Click the patch ID number. The system displays details about the patch. In addition, you can view the Read Me file before downloading the patch.
Click Download. Follow the instructions on the screen to download, save, and install the patch files.
The Oracle website contains links to all Oracle user and reference documentation. You can view or download a single document or an entire product library.
To get user documentation for Oracle Health Sciences applications, go to the Oracle Health Sciences documentation page at:
http://www.oracle.com/technetwork/documentation/hsgbu-154445.html
|
Note: Always check the Oracle Health Sciences Documentation page to ensure you have the latest updates to the documentation. |
To get user documentation for other Oracle products:
Go to the following web page:
http://www.oracle.com/technology/documentation/index.html
Alternatively, you can go to http://www.oracle.com, point to the Support tab, and then click Documentation.
Scroll to the product you need and click the link.
Click the link for the documentation you need.
The following text conventions are used in this document:
| Convention | Meaning |
|---|---|
| boldface | Boldface type indicates graphical user interface elements associated with an action, or terms defined in text or the glossary. |
| italic | Italic type indicates book titles, emphasis, or placeholder variables for which you supply particular values. |
monospace |
Monospace type indicates commands within a paragraph, URLs, code in examples, text that appears on the screen, or text that you enter. |