| Oracle® Argus Safety Japan Administrator's Guide Release 8.1 E75839-01 |
|
 Previous |
 Next |
This document describes the steps for installing and configuring the components of the Argus Console (Japanese) application.
This document is intended for administrators of the Oracle Argus Safety Japan (Argus J) Safety system for configuring Argus Safety.
This guide contains the following chapters:
Chapter 2, "Business Configuration"
Chapter 3, "System Configuration"
Chapter 4, "Code List Configuration"
This product is part of the Oracle Health Sciences Safety Suite, an integrated solution for end-to-end vigilance from adverse event management to signal management, through the entire lifecycle of a medicinal product from clinical trials to post-marketing surveillance.
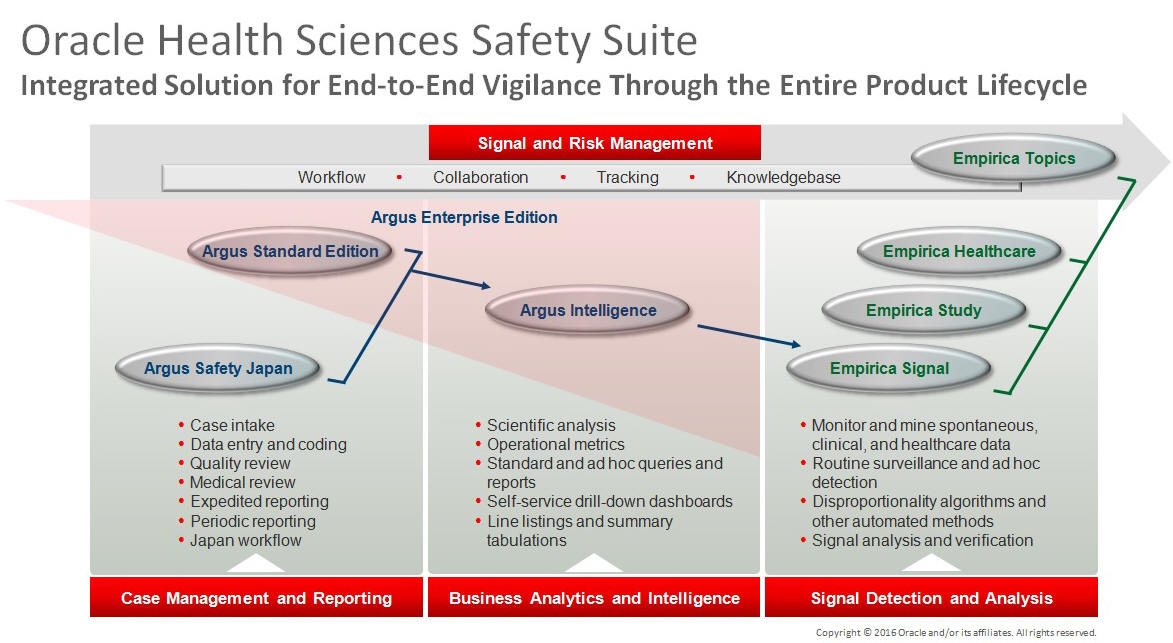
The Oracle Health Sciences Safety Suite consists of the following components:
Oracle Argus Standard Edition: Manage and report adverse events through a workflow including case intake, data entry, coding, quality review, medical review, expedited reporting, and periodic reporting. Modules include Oracle Argus Safety, Oracle Argus Interchange, Oracle Argus Affiliate, Oracle Argus Dossier, Oracle Argus Unblinding, and the Oracle Health Sciences Adverse Event Integration Pack for Oracle Health Sciences InForm and Oracle Argus.
Oracle Argus Enterprise Edition: In addition to managing the adverse event workflow and reporting, employ a powerful and flexible business analytics and intelligence platform for both scientific analysis and operational metrics. Modules include Oracle Argus Analytics, Oracle Argus Insight, Oracle Argus Mart, Oracle Argus Safety, Oracle Argus Interchange, Oracle Argus Affiliate, Oracle Argus Dossier, Oracle Argus Unblinding, and the Oracle Health Sciences Adverse Event Integration Pack for Oracle Health Sciences InForm and Oracle Argus.
Oracle Argus Safety Japan: Manage and report adverse events in Japan, and connect the global and local workflows using a single database.
Oracle Health Sciences Empirica Topics: Manage and document safety signals through a workflow including validation, prioritization, assessment, confirmation/refutation, and resulting actions.
Oracle Health Sciences Empirica Study: Detect and analyze safety signals in clinical trial data including adverse events, clinically significant labs, electrocardiograms, vital signs, and shifts from baseline.
Oracle Health Sciences Empirica Signal: Detect and analyze safety signals in post-marketing spontaneous adverse reaction data including public health authority databases and/or private inhouse databases such as Oracle Argus.
Oracle Health Sciences Empirica Healthcare Analysis: Evaluate safety signals in healthcare data including electronic medical records and administrative claims, and support pharmacoepidemiology, comparative effectiveness analysis, and health economics and outcomes research.
For more information on Argus Safety, visit the Oracle Health Sciences Safety suite page at:
http://www.oracle.com/goto/pharmacovigilance.html
For information about Oracle's commitment to accessibility, visit the Oracle Accessibility Program website at http://www.oracle.com/pls/topic/lookup?ctx=acc&id=docacc.
Access to Oracle Support
Oracle customers that have purchased support have access to electronic support through My Oracle Support. For information, visit http://www.oracle.com/pls/topic/lookup?ctx=acc&id=info or visit http://www.oracle.com/pls/topic/lookup?ctx=acc&id=trs if you are hearing impaired.
This section lists the manuals for Oracle Argus products. You can order printed manuals from the Oracle iStore.
Oracle Argus Documentation
The documentation set includes:
Argus Safety Affiliate User Guide
Argus Safety Administrator's User Guide
Argus Safety Dossier User Guide
Argus Safety Interchange User Guide
Argus Safety Installation Guide
Argus Safety Service Administrator Guide
Argus Safety Flexible Aggregate Reporting Extensibility Guide
Argus Safety BIP Aggregate Reporting User's Guide
Argus Safety User's Guide
Argus Safety Unblinding User Guide
Argus Safety Minimum Security Configuration Guide
Argus Safety Japanese Administrator's Guide
Argus Interchange Japanese User's Guide
Argus Safety Japanese User's Guide
The following text conventions are used in this document:
| Convention | Meaning |
|---|---|
| boldface | Boldface type indicates graphical user interface elements associated with an action such as Buttons, Dialog boxes, Check boxes, Combo boxes, Drop-down lists, Labels, Option (Radio) buttons, Tabs, Text boxes, etc. |
| "between quotation marks" | Information that may appear as-is on screen, or information provided by the user. |
| Note | Information that should be noted before proceeding with the instructions. |
| Important | Important information that must be noted to ensure accurate, reliable, or safe behavior of the system. |
| Tip | Information that enables easier completion of the current task or helps in completing other tasks. |
| Bold Underline | Link indicating that additional "pop-down" information is available. |
| ALL CAPITALS | Keyboard keys |
| Initial Capitals | Names of user interface elements, modules, applications, proper nouns, etc. |