Entering Protocol Data
This topic provides overviews of protocol data entry, animal subject (IACUC) and human subject (IRB) tasks, and discusses how to enter protocol data.
|
Page Name |
Definition Name |
Usage |
|---|---|---|
|
GM_PCL_VRSN |
Enter overall version information that is pertinent to the administration of the protocol, and then submit the version. |
|
|
Faculty Advisor Details Page |
GM_PCL_PI_DTLS |
Enter the name of and statement for the faculty advisor. |
|
Study Location Page |
GM_PCL_LOC |
Select the code for the study location. This page is available only for human protocols. |
|
Adverse Event Details Page |
GM_PCL_ADVRS_EVNT |
Enter a description of the adverse event. |
|
Assurance Information Page |
GM_PLC_ASSUR_INFO |
Enter an assurance ID, and approval and expiration dates. This page is available only for animal protocols. |
|
GM_PCL_PROF |
Enter role and affiliation details for personnel who are associated with the protocol. |
|
|
Details Page |
GM_PCL_PROF_DTLS |
Enter departmental details, such as phone and email information, for personnel who are associated with the protocol. See Personnel Page |
|
Training Page |
GM_PCL_PROF_TRN |
Enter all relevant training that each person received, including training date and a description of the training. This page is available only for Animal protocols. See Personnel Page |
|
Notes Page |
GM_PCL_PROF_NOTES |
Enter notes about associated personnel and the person's relationship with the protocol. See Personnel Page |
|
GM_PCL_SUB |
Enter all data that is related to the subjects who are required to conduct this particular protocol project. This page is available only for human protocols. It is hidden for animal protocols. |
|
|
GM_PCL_SUB_JUST |
Enter details about the justification for the use of subjects for the protocol. |
|
|
Certification of Compliance Page |
GM_PCL_SUB_CERT |
Enter a title and description for the certification of compliance. See Subjects Page |
|
Consent and Compensation Page |
GM_PCL_SUB_CONS |
Enter contact and communication details for consent and compensation information that is required to be documented and provided to subjects. See Subjects Page |
|
Subject Risks and Benefits Page |
GM_PCL_SUB_RIBE |
Enter a detailed explanation of the consent forms that the subjects sign. If the subject type is Children, then enter specifics for obtaining consent from children. The attachment of the actual consent form is also required. See Subjects Page |
|
GM_PCL_ANM |
Enter all data that is related to the animal subjects that are required to conduct this particular protocol project. This page is available only for animal protocols. It is hidden for human protocols. |
|
|
USDA Classification Page |
GM_PCL_ANM_USDA |
Enter details about the USDA (United States Department of Agriculture) classification. See Animals Page |
|
GM_PCL_ANM_JUST |
Enter details about the justification for the use of animal subjects for the protocol. |
|
|
Facilities and Care Page |
GM_PCL_ANM_CERT |
Enter detailed information about the facilities that are being used for this protocol. See Animals Page |
|
GM_PCL_PROC |
Enter detailed information about the purpose, methods, and procedure that are associated with the protocol. |
|
|
GM_PCL_OTHR_DTL |
Enter detailed information about the procedures that are used in this protocol. |
|
|
GM_PCL_FDA |
Enter detailed information about the FDA approval that is associated with this protocol. |
|
|
GM_PCL_HAZA |
Enter detailed information about hazardous chemicals or materials that are used in this protocol. |
|
|
GM_PCL_EUTH |
Enter detailed information about the method of euthanasia or disposition that is used in this protocol. |
|
|
GM_PCL_VRSN_ATT |
Attach files that are associated with the protocol. |
The Version Information component captures the majority of the protocol data that is required for the specific type of research involving human or animal subjects. Each subheading and table in this section represents a logical grouping of the data fields required in protocol management. In this component, the tabs represent the sub-groupings. The information in this component is created from a new proposal or copied from another version.
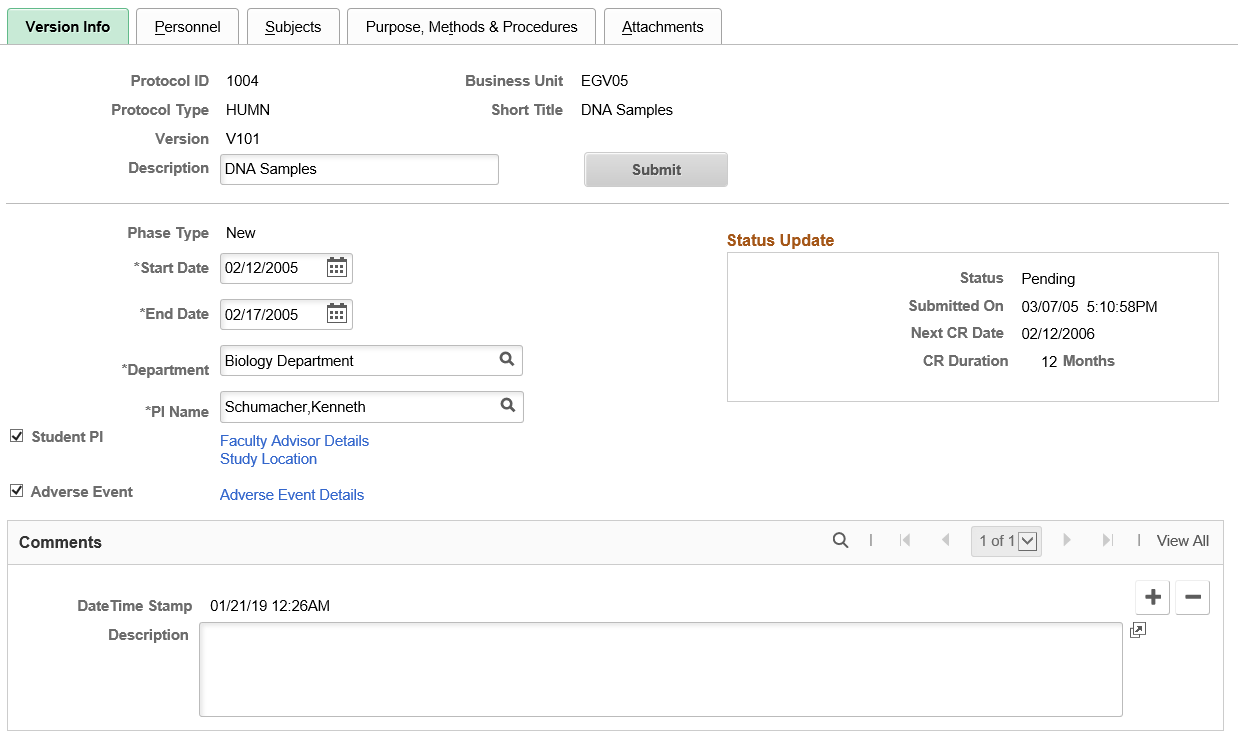
Use the Version Info (version information) page (GM_PCL_VRSN) to enter overall version information that is pertinent to the administration of the protocol, and then submit the version.
Navigation:
This example illustrates the fields and controls on the Version Info page. You can find definitions for the fields and controls later on this page.
Field or Control |
Description |
|---|---|
Submit |
Click the Submit button to submit the protocol. On Submit, the status of the protocol changes to Approved. |
Phase Type |
Displays the phase type. Values include:
|
Start Date |
Select the anticipated start date of the protocol project. This date typically matches or precedes the proposal start date . The disposition date, however, must precede the start date because the protocol approval is required before the actual start of the project. Note: Changing the start date calculates the next Continuing Review date. |
End Date |
Select the anticipated end date of the protocol project. This date is the same as the expiration date of the protocol. |
PI Name (principal investigator) |
Select the name of the employee who owns this particular proposal. In most cases, this individual is the PI on the PeopleSoft Grants proposal that is being drafted, with which this protocol will be associated. Entering the PI name will provide a default value for the Department field. |
Department |
Select the department that is associated with the protocol. |
Student PI (student principal investigator) |
Select this option if the PI is a student. If it is selected, then the Faculty Advisor Details link appears. |
Faculty Advisor Details |
Click the Faculty Advisor Details link to access the Faculty Advisor Details page. The following elements appear:
|
Study Location |
Select a study location code from the list of available values. This option is available only for human protocols. |
Adverse Event |
Select this option to indicate an adverse event. If it is selected, then the Adverse Event Details link appears. |
Adverse Event Details |
Click to access the Adverse Event Details page. Enter a description of the adverse event. |
Assurance Information |
Click to access the Assurance Information page, where you can enter an assurance ID, and approval and expiration dates. This option is available only for animal protocols. |
Status Update
Field or Control |
Description |
|---|---|
Status |
Displays the current protocol status. Values include
|
Submitted On |
Displays the date and time the protocol was submitted. The date and time is generated by the system when you click the Submit button on this page. |
Next CR Date (next continuing review date) |
Displays the date of the next continuing review. The system automatically supplies the next continuing review date by incrementing the number of months defined by the user on the Proposal Award setup page, from the start date. |
CR Duration (continuing review duration) |
Displays the duration of the review using the number of months set up for human or animal types of protocols at the business unit level. |
Comments
This is a free-form comment section for capturing any other relevant information. Add multiple comments with a date and time stamp.
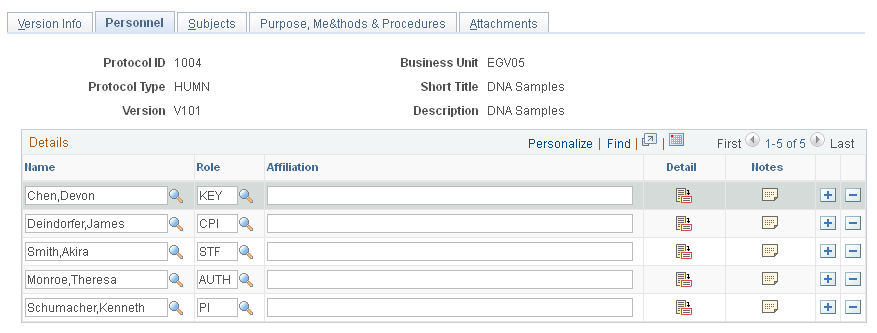
Use the Personnel page (GM_PCL_PROF) to enter role and affiliation details for personnel who are associated with the protocol.
Navigation:
This example illustrates the fields and controls on the Personnel page. You can find definitions for the fields and controls later on this page.
Details
Field or Control |
Description |
|---|---|
Role |
Select the role type of the person who is associated with this version. |
Affiliation |
Enter the person's academic, institutional, or corporate affiliation. |
Detail |
Click to enter departmental, email, and telephone information for this person. |
Training |
Click to enter all relevant training that each person received, including training date and a description of the training. Add multiple rows to capture all experience. The protocol system uses department-level security. This option is available only for animal protocols. |
Notes |
Enter notes specific to each person. |
Use the Subjects page (GM_PCL_SUB) to enter all data that is related to the subjects who are required to conduct this particular protocol project.
Navigation:
This example illustrates the fields and controls on the Subjects page. You can find definitions for the fields and controls later on this page.
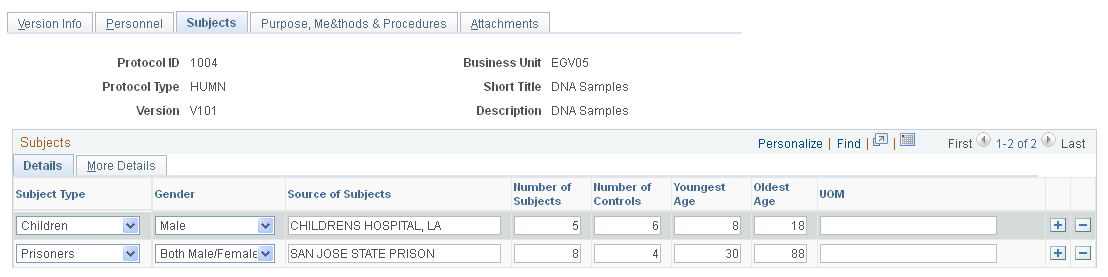
This page is available only for human protocols. It is not available for animal protocols. This page captures all data that is related to the subjects who are required to conduct this particular protocol project.
Details Tab
Field or Control |
Description |
|---|---|
Subject Type |
Select a subject type from the available values. The subject type determines which type-specific fields become viewable and actionable in the secondary pages. Values include:
You can add each subject type only once in the grid. Note: Prisoners need a prisoner representative. Children need a child advocate. |
More Details Tab
Field or Control |
Description |
|---|---|
Justification |
Click to enter a full justification for the subject type to be used for the research. |
Certification of Compliance |
Click to enter a title and explanation of the certification for the research. |
Consent and Compensation |
Click to enter contact and communication details for consent and compensation information that is required to be documented and provided to subjects. |
Subject Risks and Benefits |
Click to enter a description of the risk or benefit to be elaborated. Risks may include infection, use of placebos, expected discomfort, and known hazards. Benefits may include remission of illness or improved medical condition. Multiple rows can be added. |
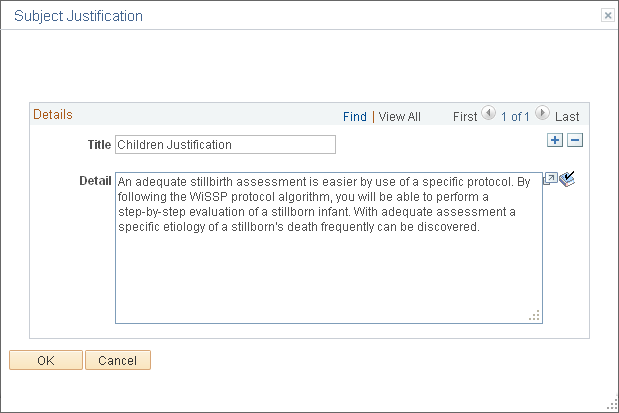
Use the Subject Justification page (GM_PCL_SUB_JUST) to enter details about the justification for the use of subjects for the protocol.
Navigation:
Click the Justification button on the Subjects page.
This example illustrates the fields and controls on the Subject Justification page. You can find definitions for the fields and controls later on this page.
Field or Control |
Description |
|---|---|
Require Fetuses Ex Utero |
Select this option if the child subject is an ex utero fetus. This option appears only if Fetuses is selected as the subject type. If you select this option, then enter certification of compliance information on the Certification of Compliance page. If this option is not selected, then the Require Dead Fetuses option becomes available. |
Field or Control |
Description |
|---|---|
Require Dead Fetuses |
Select this option if the child subject is a dead fetus. If you select this option, then enter certification of compliance information on the Certification of Compliance page. If this option is not selected, then the Require Fetuses Ex Utero option becomes available. |
Details
Field or Control |
Description |
|---|---|
Title |
Enter a short description of the justification. Add additional rows as required. |
Field or Control |
Description |
|---|---|
Justification |
Enter a full justification for involving the particular subject type for this protocol research. |
Use the Animals page (GM_PCL_ANM) to enter all data that is related to the animal subjects that are required to conduct this particular protocol project.
Navigation:
This example illustrates the fields and controls on the Animals page. You can find definitions for the fields and controls later on this page.
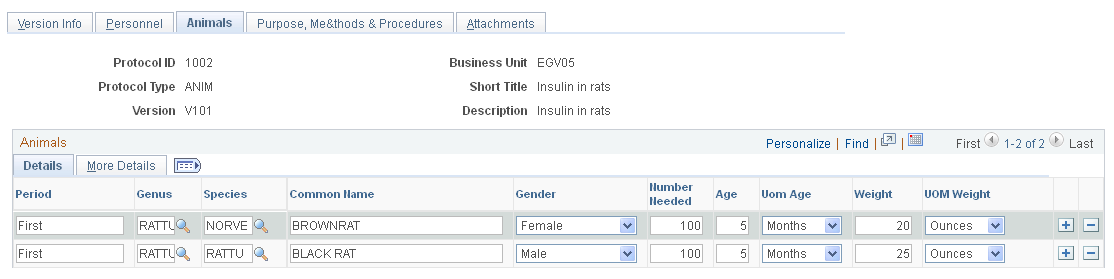
This page is available only for animal protocols. It is not available for human protocols. This page captures all data that is related to the animals that are required for this particular protocol.
Details Tab
Field or Control |
Description |
|---|---|
Period |
Enter which period this row's information applies to. This is a free-form text field. |
Genus |
Enter the animal's division of family (for example, Rattus). |
Species |
Enter the animal's division of genus (for example, Rattus norvegicus). |
Strain, Subspecies, or Breed |
Enter the animal's strain, subspecies, or breed. |
Common Name |
Enter the animal's common name of the animal (for example, Brown Rat). |
More details Tab
Field or Control |
Description |
|---|---|
Age |
Enter the animal's age. |
UOM (unit of measure) |
Enter whether the age is indicated in terms of minutes, hours, days, months, or years. |
Use the Animal Justification page (GM_PCL_ANM_JUST) to enter details about the justification for the use of animal subjects for the protocol.
Navigation:
Click the Justification button on the Animals page.
This example illustrates the fields and controls on the Animal Justification page. You can find definitions for the fields and controls later on this page.
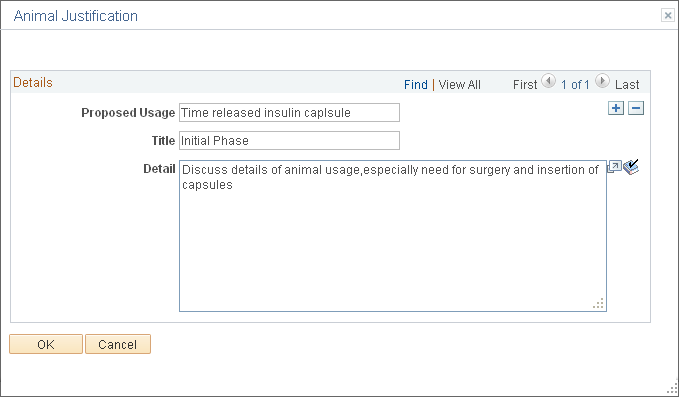
Details
Field or Control |
Description |
|---|---|
Proposed Usage |
Enter an explanation of how you plan to use the animals. |
Title |
Enter a short description of the justification (for example, the rationale for using live animals). |
Field or Control |
Description |
|---|---|
Detail |
Enter a justification for this protocol research. |
Use the Purpose, Methods & Procedure page (GM_PCL_PROC) to enter detailed information about the purpose, methods, and procedure that are associated with the protocol.
Navigation:
This example illustrates the fields and controls on the Purpose, Methods & Procedures page. You can find definitions for the fields and controls later on this page.
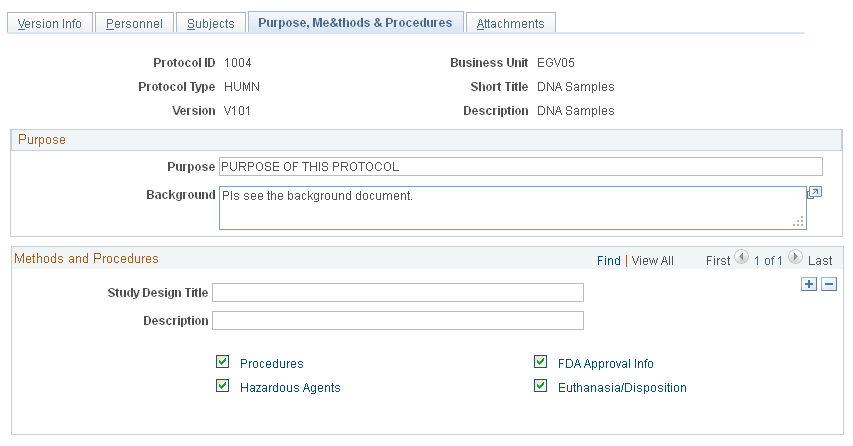
Purpose
Field or Control |
Description |
|---|---|
Purpose |
Enter the purpose of the research. |
Background |
Enter background related information; for example, how the research came about. |
Methods and Procedures
Field or Control |
Description |
|---|---|
Study Design Title |
Enter a short description of the hypothesis, research questions, statistics, or other topics that are related to the research. Add additional rows and attach documents as needed. |
Description |
Enter study design details. |
Procedures |
Select this option and click the link to go to the Procedures page. |
Hazardous Agents |
Select this option and click the link to access the Hazardous Agents page. |
FDA Approval Info (FDA approval information) |
Select this option and click the link to access the FDA Approval page. |
Euthanasia/Disposition |
Select this option and click the link to access the Euthanasia/Disposition page. |
Use the Procedures page (GM_PCL_OTHR_DTL) to enter detailed information about the procedures that are used in this protocol.
Navigation:
Click the Procedures link on the Purpose, Methods & Procedure page.
This example illustrates the fields and controls on the Procedures page. You can find definitions for the fields and controls later on this page.
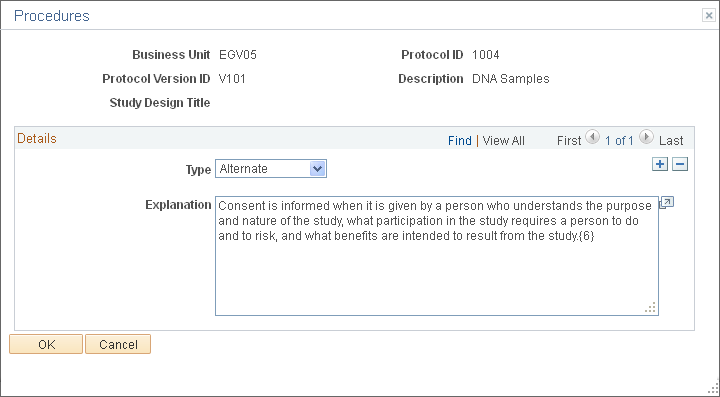
Details
Field or Control |
Description |
|---|---|
Type |
Select a type and enter an explanation of the procedure. Type values include:
|
Use the Hazardous Agents page (GM_PCL_HAZA) to enter detailed information about hazardous chemicals or materials that are used in this protocol.
Navigation:
Click the Hazardous Agents link on the Purpose, Methods & Procedure page.
This example illustrates the fields and controls on the Hazardous Agents page. You can find definitions for the fields and controls later on this page.
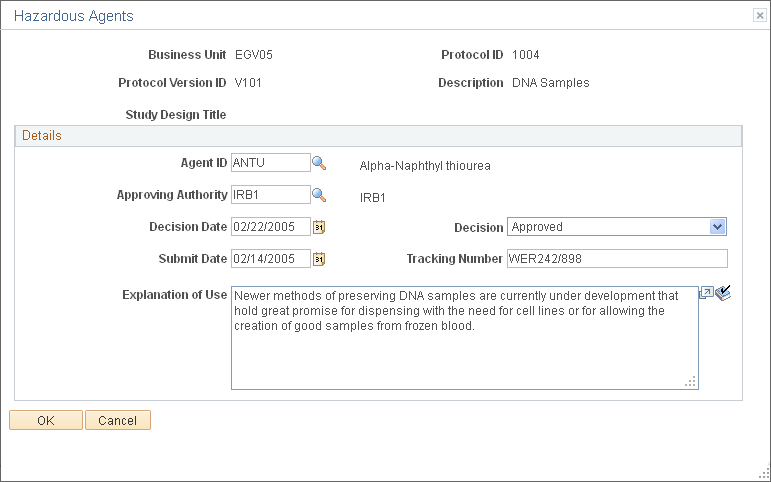
Details
Enter an explanation about the hazardous agents used in the protocol.
Field or Control |
Description |
|---|---|
Agent ID |
Select the Agent ID. |
Approving Authority |
Select the approving authority. |
Decision Date |
Enter the date the decision was made about the use of the hazardous agent. |
Decision |
Select the Decision status from Approved, Pending, and UnApproved. |
Submit Date |
Enter the date the hazardous agent was submitted to the approving authority. |
Tracking Number |
Enter the number used to track the hazardous agent. |
Explanation of Use |
Enter a description of how you use the hazardous agent. |
Use the FDA Approval page (GM_PCL_FDA) to enter detailed information about the FDA approval that is associated with this protocol.
Navigation:
Click the FDA Approval Info link on the Purpose, Methods & Procedure page.
This example illustrates the fields and controls on the FDA Approval page. You can find definitions for the fields and controls later on this page.
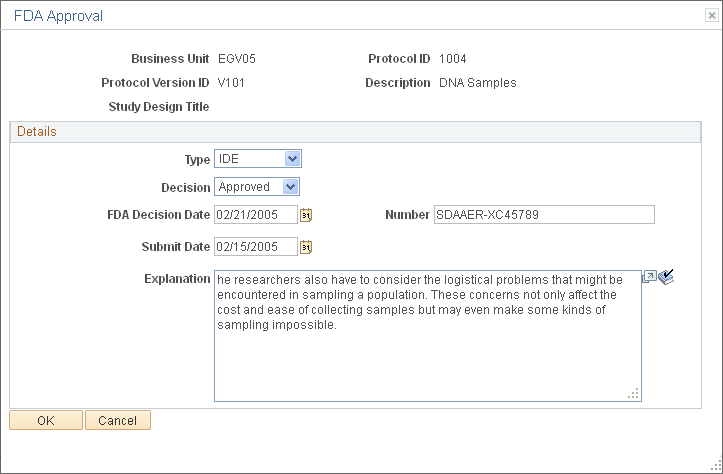
Details
Field or Control |
Description |
|---|---|
Type |
Select IDE (investigational device exemption) or IND (investigational new drug). |
Field or Control |
Description |
|---|---|
Decision |
Select Approved, Pending, or Unapproved. |
Field or Control |
Description |
|---|---|
FDA Decision Date |
Enter the date on which the FDA approved or disapproved the decision. |
Field or Control |
Description |
|---|---|
Number |
Enter the FDA decision number. |
Field or Control |
Description |
|---|---|
Submit Date |
Enter the date on which the study plan was submitted for review. |
Explanation |
Describe the details of the FDA decision. |
Use the Euthanasia/Disposition page (GM_PCL_EUTH) to enter detailed information about the method of euthanasia or disposition that is used in this protocol.
Navigation:
Click the Euthanasia/Disposition link on the Purpose, Methods & Procedure page.
This example illustrates the fields and controls on the Euthanasia/Disposition page. You can find definitions for the fields and controls later on this page.
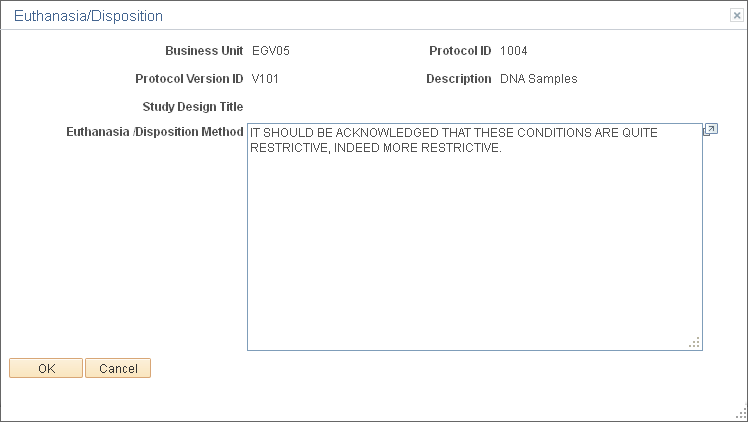
Field or Control |
Description |
|---|---|
Euthanasia/Disposition Method |
Enter an explanation of the euthanasia or disposition method. |
Use the Protocol - Attachments page (GM_PCL_VRSN_ATT) to attach files that are associated with the protocol.
Navigation:
Field or Control |
Description |
|---|---|
Requests |
The system automatically generates the number of requests. |