- User's Guide
- Oracle Argus Affiliate Users
- Submitting Reports
Submitting Reports
- Select Report Distribution from the Local
Affiliate menu to open the Report Distribution page.
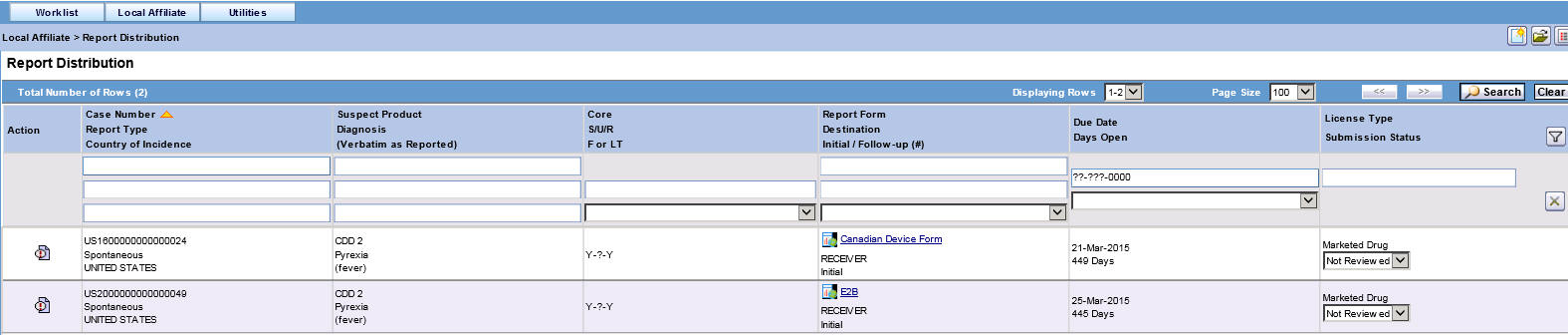
Report Distribution Fields
The following table lists and describes the fields on the Report Distribution page.
Field Description Action
Enables you to view and select the different options available as action items.
Case Number
Enables you to search for a case based on its case number.
Report Type
Displays the type of report.
Country of Incidence
Displays the name of the country where the adverse event occurred.
Suspect Product
Displays the name of the suspect product.
Diagnosis (Verbatim as reported)
Displays the diagnosis made for the event.
Core
Displays the core labeling made for the event.
S/U/R
Displays whether the case is serious unrelated or related.
F or LT
Displays if the event is Fatal or Life-Threatening.
Report Form
Displays the name of the report in a link.
Click the link to view the report in a PDF.
Destination
Displays the destination name.
Initial/Follow-up (#)
Displays if the report is an initial report or a follow-up report.
Date Due
Displays the date when the report is due.
Days Open
Displays the days since when the report has been open.
License Type
Displays the license type of the report.
Submission Status
Displays the submission status for the report.
- Use the standard filters provided for Case Number, Report Type, Country of Incidence, Suspect Product, Diagnosis, and so on to filter to locate the required report.
- Locate the report to be submitted and click the icon associated with the report in order to view the available options.
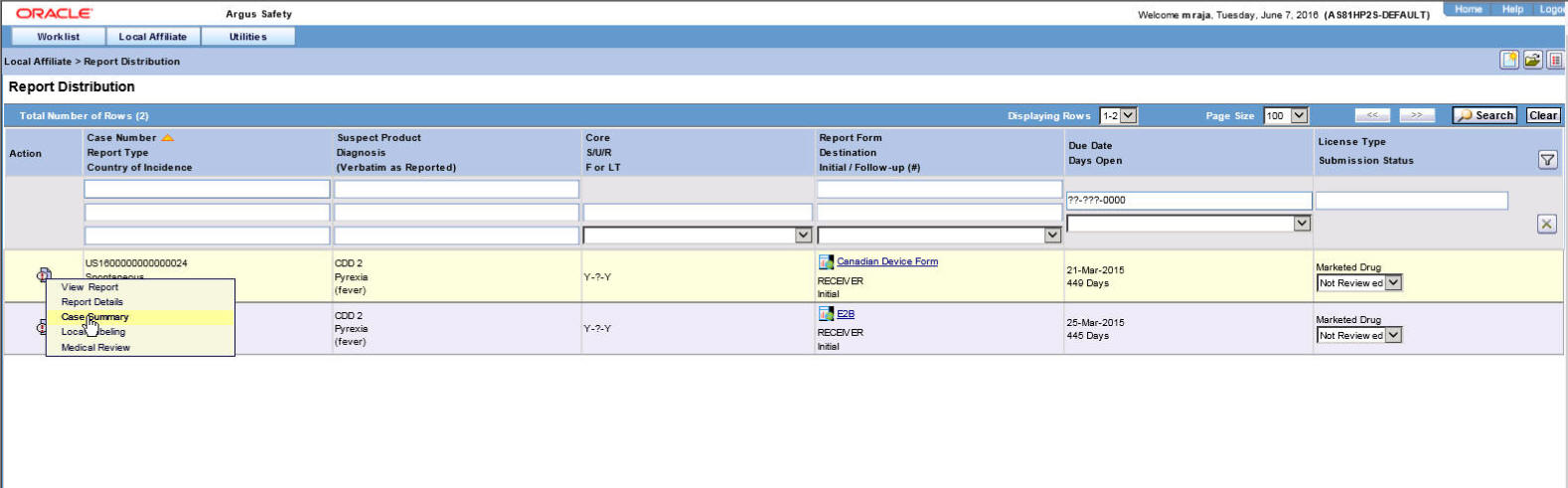
Descriptions of the Action Items
The following table lists and describes the available action items.
Field Description View Report
Displays the selected report in a PDF.
Report Details
Enables you to view the report details associated with the report.
Case Summary
Enables you to view a summary of the selected case as shown in the table below.
Local Labeling
Enables you to determine whether labeling has been assessed for the case.
Medical Review
Displays the Medical Review screen of Argus.
The following table describes the meaning of each action item.
Action Item Description 
This report has been scheduled/generated and it is past its due date of submission.
This report has been scheduled and saved.

This report has been scheduled and generated.

This report has been routed and approved by a user.
Case Summary Field Descriptions
The following is an illustration of the Case Summary:
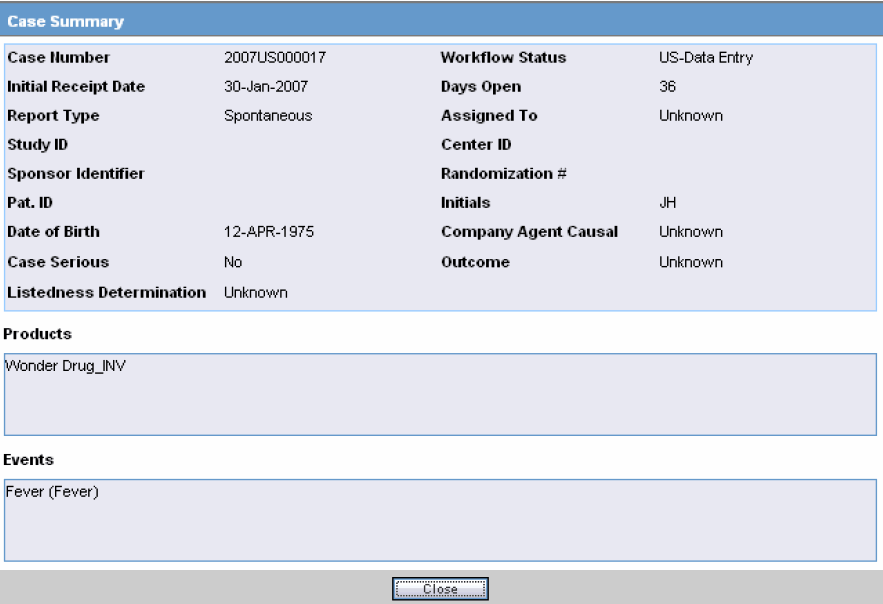
The following table lists and describes the Case Summary fields.
Field Description Case Number
Displays the case number
Workflow Status
Displays the workflow status of the case
Initial Receipt Date
Displays the Initial Receipt Date of the case
Days Open
Displays the number of days the case has been opened.
This is calculated by the difference between the Initial Receipt Date and the System Date (Current Date)
Report Type
Displays the Report Type.
Assigned To
Displays the individual that the case was assigned to.
Study ID
Displays the Study ID of the case
Center ID
Displays the Center ID of the case
Sponsor Identifier
Displays the Sponsor Identifier of the case
Randomization #
Displays the Randomization # of the case
Pat. ID
Displays the Patient ID
Initials
Displays the Initials of the patient
Date of Birth
Displays the Date of Birth of the patient
Company Agent Causal
Displays the whether the case was Company Agent Causal or not.
Case Serious
Displays whether the case was serious or not.
Outcome
Displays the outcome of the case.
Listedness Determination
Displays the Listedness status of the case
Products
Displays the Suspect Products associated with the case.
Events
Displays the Events associated with the case.
- In the Submission Status list of the required report, select
Submit.
You can submit multiple reports at a time by selecting Submit for the required reports.
- Click Process to open the Report Submission Information dialog box.
- Enter any remarks in Note and click OK.
- The report(s) opens and a list of submitted reports is generated.
Parent topic: Oracle Argus Affiliate Users