Report Output Field Mappings
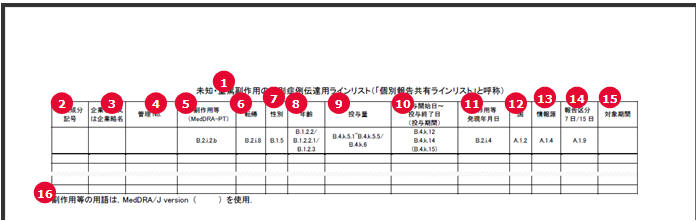
| # | Field Name | Description |
|---|---|---|
|
1 |
Serious, Unlisted AE Individual Case Communication Line List |
This represents the title of the paper form. It is a fixed text to be printed in the top center of the Report Output table. |
|
2 |
Clinical Compound Number |
Clinical Compound Number of the report/case. For Clinical and Domestic studies, it prints J.11 (mhlwcompoundnum) element value from the expedited/E2B report. For Foreign Ichihen studies, it prints the Clinical Compound Number from the License Configuration for the Japanese licenses present in Case Form > Event Assessment tab. |
|
3 |
Company name or Company name abbreviation |
If you have specified the Company Name to be printed in the Individual Report Common Line List configuration tab, that text is printed as it is for all the event rows. Else, it is left blank. |
|
4 |
Management No. (Case No) |
The Argus Case ID number for the corresponding event is printed in this field. |
|
5 |
Adverse Event (MedDRA-PT) |
This field represents the name of the Serious and Unlisted MedDRA J Term (B.2.1.2.b) For clinical and domestic studies: It prints MedDRA PT (J) term based on the B.2.1.2.b (REACTIONMEDDRAPT) value from the Expedited/E2B report. For decoding the MedDRA PT code from the Expedited/E2B report into the J Term, latest MedDRA dictionary configured for event encoding is used. For foreign ichihen studies: It prints MedDRA PT (J) term from Case Event record. |
|
6 |
Outcome |
This field represents the Reaction Outcome (B.2.i.8) For clinical and domestic studies: It prints the following Japanese description text for the E2B code values from the B.2.i.8 (REACTIONOUTCOME) value from the Expedited/E2B report. For foreign ichihen studies: It prints the Event Outcome (J) value from the Case Event record. If no value is available in the case, then print NULL. The Reaction Outcome footer in PSR Form 4 has E2B (R3) code for Reaction Outcome against E2B (R2) Code 6. |
|
7 |
Gender |
This field represents the Patient Gender (B.1.5). For clinical and domestic studies: It prints the E2B M2 Japanese description based on the B.1.5 (PATIENTSEX) value from the Expedited/E2B report or the case. If B.1.5 is not available, print Unknown For reporting categories L, M, and N, it is left blank. For foreign ichihen studies: It prints the Patient Gender (J) value from the Case Patient record. If no value is available in the case, print Unknown |
|
8 |
Age |
This field represents the Patient Age (B.1.2.2/B.1.2.2.1/B.1.2.3) It prints the Patient Age value based on the E2B logic for Patient Age elements. For example, If the Child Only check box in Case is checked, only GESTATIONPERIOD (B.1.2.2.1) is transmitted. If it is not checked, and if PATIENTONSETAGE (B.1.2.2) is specified, only it is transmitted else PATIENTAGEGROUP (B.1.2.3) is transmitted. For PATIENTONSETAGE, age (B.1.2.2a) value is printed followed by the E2B M2 Japanese description of the Age Unit (B.1.2.2b) without any space in between . For PATIENTAGEGROUP, E2B M2 Japanese description of B.1.2.3 is printed. For GESTATIONPERIOD, TBD is printed as fixed text followed by the Gestation period (B.1.2.2.1a) value and the E2B M2 Japanese description of the unit (B.1.2.2.1b) without any space in between. For PATIENTONSETAGE, if the unit value is Decade (B.1.2.2b = 800), special printing rule is followed. |
|
9 |
Dose |
This field represents the Product Dose Information (B.4.k.5.1~B.4.k.5.5/B.4.k.6) In case of multiple dosages for the Seiyakukyo product(s), all the dosages are printed in the same cell, in the same order as in the Expedited/E2B report or as per the Product and Dosages order in the case. Each new dose information is printed after leaving a blank line and in alignment with the corresponding dose Start/Stop date value which is printed in the next column. For Clinical and Domestic studies, each dose information is printed using the following format: [B.4.k.5.1][B.4.k.5.2 (using E2B M2 Japanese description)][B.4.k.5.3]?/[B.4.k.5.4][B.4.k.5.5(using E2B M2 Japanese description)] - TBD([B.4.k.6(If this does not exist, then its braces for this value are not printed. However, a blank line is left to align the Dosage Information with the corresponding dosage dates values in the adjacent column.)]) For foreign ichihen studies, each Dose Information is printed in the following format using the value directly from the Case Product Dosage Record: [Dose][Dose Unit (J)]Code List/Dosage Frequency/[Number]TBD/[Unit Number][Unit (J)]([Dose Description (J)]) - If this does not exist, then its braces for this value is not printed. However, a blank line is left to align the Dosage Information with the corresponding dosage dates values in the adjacent column. |
|
10 |
Dose Start Date ~ Dose Stop Date (Dose interval) |
This field represents the Product Dosage Start/Stop DatesB.4.k.12B.4.k.14(B.4.k.15) In case of multiple dosages for the product(s), all the dosage dates are printed in the same cell, in the same order as in the Expedited/E2B report or in the case. Each new Dosage Date is printed after leaving a blank like and in alignment with the corresponding dosage information value which is printed in the previous column. For foreign ichihen studies, each dosage date is printed using the Dose Start Date, Dose End Date, and Dose Duration values from the Case Product Dosage record in the same format as specified for Clinical and Domestic studies. Dose duration value is converted from seconds into appropriate unit using the same logic as used in E2B for B.4.k.15. |
|
11 |
AE, etc. onset date |
This field represents the Reaction Onset Date (B.2.i.4) For Clinical and Domestic studies, it prints the REACTIONSTARTDATE (B.2.i.4a and B.2.i.4b) value. For foreign ichihen studies, it prints the Event Onset Date from Case Event Record. |
|
12 |
Country |
This field represents the Country of Incidence (A.1.2) For Clinical and Domestic studies, it prints the E2B M2 Japanese description based on the A.1.2 (OCCURCOUNTRY) value from the Expedited/E2B report. For foreign ichihen studies, it prints the Country Name (J) value from the Case Record. |
|
13 |
Information Source |
This field represents the Report Type (A.1.4) For clinical and domestic studies, it prints the E2B M2 Japanese description based on the A.1.4 (REPORTTYPE) value from the Expedited/E2B report. For foreign ichihen studies, it prints the Report Type (J) value from the Case Record. |
|
14 |
Report Type7 days/15 days |
This field represents the meeting Expedited Report Criteria (A.1.9) For clinical and domestic studies, it prints 7 days or 15 days, if the value of A.1.9 (FULFILLEXPEDITECRITERIA) is 1 or 2 respectively, in the Expedited/E2B report. For Foreign Ichihen studies, it is left blank. |
|
15 |
Subject timeframe |
This field represents the Reporting Timeframe considered for the AE. It prints the Seiyakukyo report current timeframe. The same values are printed in all the rows. |
|
16 |
For the AE terms, MedDRA/ J version ( ) is used. |
This field represents the MedDRA J version used for this report. It prints the MedDRA version as currently configured in the application for event encoding. This is repeated at the end of each Clinical Compound Number section. |
|
17 |
Any additional columns added by the user in configuration (applicable only to CSV output) |
Study Name: For clinical and domestic studies, it prints the value of A.2.3.2 (SPONSORSTUDYNUMB) from the Expedited/E2B reports. For foreign ichihen studies, it prints Study ID (J) from the case record. If Study Name (J) is not available in the case, print the corresponding English value. Blinded / Not Blinded: For all types of cases, it prints the Japanese text - Blinded or Not Blinded, based on the Case Form > Study Information > Study Type field value for the corresponding case. SOC: For clinical and domestic studies, it prints the MedDRA SOC (J) Term based on the B.2.1.2.b (REACTIONMEDDRAPT) value from the Expedited/E2B report. For decoding the MedDRA PT code from the Expedited/E2B report into J Term, latest MedDRA dictionary configured for Event Encoding is used.For foreign ichihen studies, it prints the MedDRA SOC (J) Term from the Case Event record. NOTE: Since L, M, and N category cases are not included in the report, this data will no longer be left blank for these categories. LLT: For clinical and domestic studies, it prints the MedDRA LLT (J) Term based on the B.2.1.2.b (REACTIONMEDDRALLT) value from the Expedited/E2B report. For decoding the MedDRA PT code from the Expedited/E2B report into J Term, latest MedDRA dictionary configured for event encoding is used. For foreign ichihen studies, it prints the MedDRA LLT (J) Term from the Case Event record. NOTE: Since L, M, and N category cases are not included in the report, this data will no longer be left blank for these categories. MedDRA Version:For clinical and domestic studies, it prints the MedDRA version as used in B.2.i.2.a (REACTIONMEDDRAVERSIONPT) from the Expedited/E2B report. For foreign ichihen studies, it prints the MedDRA version from the case for that Event Encoding. NOTE: Since L, M, and N category cases are not included in the report, this data will no longer be left blank for these categories. |
|
17 |
Any additional columns added by the user in configuration (applicable only to CSV output) |
Report Submission Date: For Clinical and Domestic studies, it prints the corresponding Expedited/E2B Report Submission date after converting it into Japanese timezone based on the Argus J > Reporting > Offset from GMT Common Profile Switch that is used to calculate Japanese date/time fields for Interchange-J (in hours). For Foreign Ichihen studies, it is left blank. Japan Information Receipt Date: For Clinical and Domestic studies, it prints J.3b (mhlwadmicsrinfoobtndatesource). For Foreign Ichihen studies, it prints the Japan Aware date. ACK Number: For Clinical and Domestic studies, it prints the eight digit PMDA ACK number received for the corresponding Expedited/E2B report. For Foreign Ichihen studies, it is left blank. Number of times this report is submitted to MHLW: For clinical and domestic studies, it prints J.5 (mhlwadmicsrmhlwcumreporttimes) element value. For Foreign Ichihen studies, it is left blank. Primary Disease: For all types of cases, it prints the Condition (J) value as present in Case Form > Patient tab > Other Relevant History section. Only those items are printed which have Primary Disease text present in the Notes (J) field. In case multiple items have to be printed, then these are printed in separate lines in the same cell. Event Intensity: For all types of cases, it prints the Case Form > Event tab > Intensity (J) field value. NOTE: Since L, M, and N category cases are not included in the report, this data will no longer be left blank for these categories. |
|
17 |
Any additional columns added by the user in configuration (applicable only to CSV output) |
Reported Causality: Only one value is printed for this field based on the Reported Causality values specified for those products in the case, whose valid Japanese licenses have the same Clinical Compound Number for which this event is being printed. Only the causality values that are related to the current event being printed are considered. The following rule is used to determine the value that is printed in the report output. Related - If any of the Reported Causalities is Reportable (Consider Expeditable to Authorities is checked in Console Code list) Not related - Else, if any of the Reported Causalities excluding Unknown is not reportable Unknown - Else, if any of the Reported Causality is Unknown No value - Else, if all the Reported Causalities are blank. NOTE: Since L, M, and N category cases are not included in the report, this data will no longer be left blank for these categories. Determined Causality: Only one value is printed this for field based on the Determined Causality values specified for those products in the case, whose valid Japanese licenses have the same Clinical Compound Number for which this event is being printed. Only the causality values that are related the current event being printed are considered. The following rule is used to determine the value that is printed in the report output. Related - If any of the determined causalities is Reportable (Consider Expeditable to Authorities is checked in Console Code list) Not Related - If any of the determined causalities is Reportable (Consider Expeditable to Authorities is checked in Console Code list) Unknown - Else, if any of the determined causalities excluding Unknown is not Reportable No Value - Else, if all the determined causalities are blank. NOTE: Since L, M, and N category cases are not included in the report, this data will no longer be left blank for these categories. |
|
18 |
Section Footer Text |
It prints the configured text as specified in Individual Report Common Line List Configuration tab > Section Footer text below the event output table right after MedDRA J Version, in a new line. This text is repeated at the end of each Clinical Compound Number section. |
|
19 |
Page Numbering |
The page numbering for the Seiyakukyo report starts from 1 for each Clinical Compound Number section independently. The page numbering for Seiyakukyo report is printed in the middle of page footer in the following format: <current page number>/<Total Pages> The page numbering for attached CIOMS reports starts from 1 for each CIOMS independently. The page numbering for attached CIOMS reports is printed in the top left corner of CIOMS report table (same as in attached CIOMS in PSURs) in the following format: <current page number>/<Total Pages> |
|
20 |
Page Size/Font |
The MS Word A4 Landscape page size is used for Seiyakukyo report. Ms Mincho font is used for the report output. |