License Configuration - Oracle Argus Safety Japan Specific Parameters
The following changes have been made for PMDA Device Reporting Support in Console:
- The following drop-down lists have been added to Console
> Business Configuration > Products and
Licenses. These drop-down lists have the following options in the
same order.
-
PMDA Device Classification 1:
High Level Controlled Medical Device (Class IV)
High Level Controlled Medical Device (Class III)
Controlled Medical Device
Generic Medical Device
Combination products (Drugs)
Combination products (Tissue-Engineered Medical Products)
Stand-alone software (Class IV)
Stand-alone software (Class III)
Stand-alone software (Class II)
PMDA Device Classification 2:
Biogenous
Specific Biogenous
Other
PMDA Device Classification 3:
Single Use Medical Device
Reiteration Use Medical Device
Figure 1-1 Console License Configuration - PMDA Device Classifications
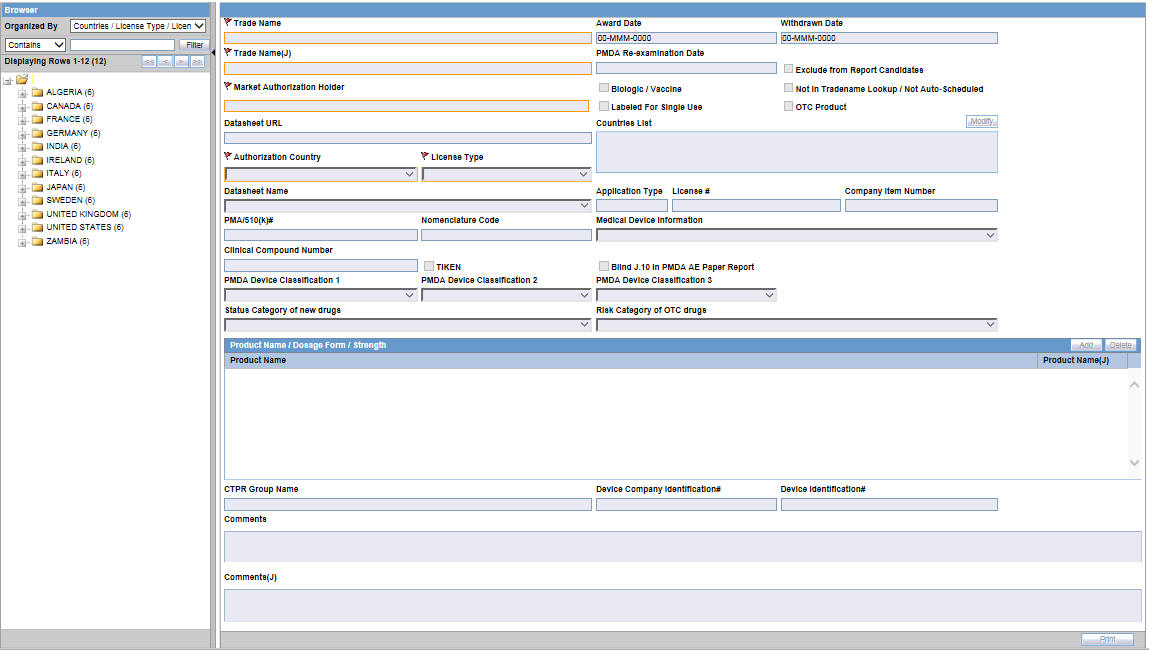
- These drop-down lists have <Blank> as the default value.
- These fields are displayed to only an Oracle Argus Safety Japan user when Japanese module is enabled.
- These fields are editable only when Authorization Country is selected as Japan and License Type is selected as either Marketed Device or Investigational Device.
- The list options are displayed in English even to the Oracle Argus Safety Japan user as this is an English base screen. The Japanese value specified for these options is used to populate them in PMDA Device Expedited Form 8 and 10.
- Medical Device Information and Clinical Compound Number have been adjusted in the user interface of the application.
- These three fields are printed in License Print PDF in three different rows, right below Clinical Compound Number field in alternate-colored rows thereafter.
- These fields are audit-logged.
- These fields are covered by the back-end PL/SQL APIs for License Configuration data table updates and audit-logging.
- A new checkbox TIKEN is available. Any changes to this checkbox value are audit logged.
- Blind J.10/J2.11 in PMDA AE Paper Report: This checkbox is disabled by default and shall be enabled only when the License country is Japan.
- Status Category of new drugs: This list captures the Status category of new drugs. The data in this list is populated based on the data in the License Category code list.
-
Risk Category of OTC drugs: This list captures the Risk Category of over-the-counter (OTC) drugs. The data in this list is populated based on the data in the Risk Category of OTC Drug code list.
These new fields available in License Configuration print for both Print and Print All options. They also support the License/Product with Licenses copy functionality. Any changes to these fields value is logged for audit.
-
- A separate Japanese Comments field is supported for the following in Console '
Business Configuration.
-
Comments (J) field has been added right below the English Comments area.
This field is displayed only to Oracle Argus Safety Japan users when Japanese module is enabled.
It is printed in the License Configuration print PDF right after the English Comments field.
It is audit-logged and is also covered by the back-end PL/SQL APIs for License Configuration data table updates and audit-logging.
-
Parent topic: Configuring Licenses