J-DSUR - Cover
Summary: The J-DSUR Cover is the cover page of the J-DSUR Line Listing, and output includes the information of the configured product. This form does not contain any particular case data.
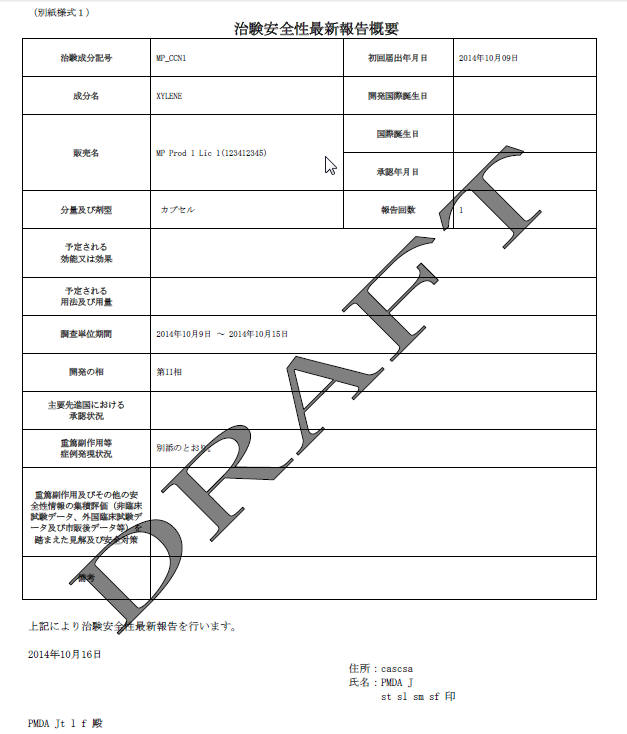
| Field Name | Description |
|---|---|
|
Clinical Study Drug Serious Adverse Events etc. Case Periodic Safety Report Form |
NA |
|
Clinical Compound Number |
This field represents the Clinical Compound Number of the selected product from Console Business Configuration/Product/License.Use ([1],[2],[3]… ) numbering format for multiple Clinical Compound Numbers. |
|
Clinical Study Plan Submit Date |
This field is a manual data entry field. If this field is changed by the user and this date is later than the "Clinical Trial for Partial Change Plan Submit Date", it displays a message that CSPSD cannot be after CTPCSD. |
|
Ingredient Name |
The generic name is entered in this field. If the license is withdrawn before the starting date of the CSPSR timeframe, the generic name is not displayed. If Japanese name is not available, this field can be left blank. |
|
Dev International Birth Date |
This field is auto-populated as per the earliest Console > Product > DIDB field for the J-DSUR products. This date is used to auto-populate the first J-DSUR Start Date on the "Frequency of the schedule" table on the right side of the UI. |
|
Trade Name |
The Trade Name is based on the selected product in the Configuration window. If there are multiple products (different amount, formulation, etc), use ([1],[2],[3]… ) numbering with the trade names. You can use Trade name (J Drug code). When the code is not available in the code lists, the code is not necessary. If there is a product that has multiple Japanese licenses, all of the trade names must be listed. This field is entered when the selected product is the marketed product (Clinical Trial for Partial Change), but if this is for the Study drug, this field is left blank. |
|
International Birth Date |
This field represents the IBD from the Code List Product. Use ([1],[2],[3]… ) numbering for multiple names. |
|
Japan Award Date |
Code List Licenses -Japan Award date Use ([1],[2],[3]… ) numbering for multiple names. All the Japanese award dates are listed in this field. The Japanese Award date can be retrieved from License award date field for Japanese license Code List. If the license is withdrawn before the starting date of the CSPSR timeframe, the Japan Award Date is not displayed. This field is entered when the selected product is the marketed product (Clinical Trial for Partial Change), but if this is for the Study drug, this field is left blank. |
|
Amount and Formulation |
This field represents the amount of the ingredients and formulation (from Dosage info) of the product. If multiple formulations are reported in the same form, enter the information for each formulation. The Strength and unit of the product, and formulation are listed in the following format:"Strength""unit" "Formulation"If the strength data is not available, only the formulation is listed. Use ([1],[2],[3]… ) numbering for multiple information. If Japanese name is not available, the section is left blank. |
|
Number of the CSPSR report sent for this ingredient |
This field denotes the total number of CSPSR report sent to PMDA including the current time. This starts from 1, 2, 3… |
|
Expected Indication |
Enter Primary Indication from the Business Configuration/Product/Primary Indication. Use ([1],[2],[3]… ) numbering for multiple Indications. If Japanese name is not available, this section is left blank. |
|
Expected Use and Dosage |
The information is not printed in this section. You need to enter the information in the Word document output. |
|
Investigational Unit Timeframe |
This field is used to enter the Time frame Start Date and Time frame End Date for this report. This denotes the Investigation timeframe for this report. |
|
Study Phase |
Enter Console J > Business Configuration > Studies > study phase J using this field. When there are multiple clinical studies that use the same Clinical Compound number, use ([1],[2],[3]… ) numbering and list each study phase of all the studies using comma separated string against each number. It only considers the studies configured as Selected Domestic Studies in the Product Selection tab. If Japanese name is not available, this section is left blank. |
|
Approval Status in major developed countries |
This field represents Approval status in major developed countries excluding Japan. The value in this field is printed based on the earliest award date of marketed licenses (excluding the hidden link licenses for these products) for the J-DSUR product(s) for configured countries in profile switch Argus J > Reporting>'Major Developed Countries for Approval Status in J-DSUR' (comma separated A2 country codes). Use numbering ([1],[2],[3]… ) for multiple products. The country is printed with its A2 country codes and Aware Date year shall be printed in YYYY format. Example: If in a JDSUR report we have 2 products P1 and P2 with P1 having 2 CCN - CCN1, CCN2 and P2 having 2 CCN - CCN3, CCN4 then approval status are printed as - ([1], [2]) US - YYYY, UK - YYYY, DE - YYYY ([3], [4]) US - YYYY, UK - YYYY, DE - YYYY Where US, UK and DE are configured country codes in the profile switch in this particular order only. Only Marketed Licenses related information are printed here. If Marketed License is not available then do not print any value for that country not even the A2 country code for that country. The values are printed in the same order as A2 codes entered by user in the common profile switch. |
|
Status of the Serious AE, etc. case occurrences |
This field is used to enter TBD (As described in the Line Listing report). |
|
Safety action based on the data analysis and safety countermeasure for the future |
This field is left empty to enter the description in the output format. |
|
Note |
This field is left empty to enter the description in the output format. |
|
From above reason, this reports Clinical Serious Case AE Periodic Reports |
NA |
|
YYYY?MM?DD? TBD |
The Date report is created in the Japanese format. It is printed as the current database date converted into Japan time zone (using the Common Profile Switch under Argus J > Reports > Offset from GMT and is used to calculate Japanese date/time fields (in hours)) on which the report is being executed. |
|
Address |
This field represents the Address of the sender (A.3.1.4a-c). Format: TBD[A.3.1.4c][A.3.1.4b][A.3.1.4a] A line break is inserted if the address is reached to the maximum printable length for the line in the print. The address line can be 3 lines in one page, and if the 3rd line reaches the maximum length, it is printed to the next page. No spaces are used between the populated values. The Address and the Name are printed from center of the page (same as PMDA Expedited Reports Name and Address print) |
|
Name |
This field represents the Sender Identifier(Company Name (J), A.3.1.3b, A.3.1.3c, A.3.1.3d, A.3.1.3e) Format: TBD[Company Name (J)] [A.3.1.3b]space[A.3.1.3e]space[A.3.1.3d]space[A.3.1.3c]The print start point of [A.3.1.3b] is the same as [Company_Name_(J)] in above line. For the second line, empty space is printed between the populated values. A line break is inserted, If A.3.1.2 reaches the maximum printable length for the line. There can be 2 lines in one page. And if the 2nd line reaches the maximum length, it is printed to the next page.. A line break is inserted, If combination of [A.3.1.3b] [A.3.1.3e] [A.3.1.3c] reaches the maximum printable length for the line. There can be 2 lines in one page. And if the 2nd line reaches the maximum length, it is printed to the next page. The SENDERMIDDLENAME is not printed if it does not exist. The Address and Name is printed from center of the page (same as PMDA Expedited Reports Name and Address print) |
|
Receiver identifier |
PMDA Receiver identifier A.3.2.2d-f in _____ section.This is printed as follows:Receiver Identifier (A.3.2.2a, A.3.2.2c, A.3.2.2d, A.3.2.2f) Format: [A.3.2.2a][A.3.2.2c]space[A.3.2.2f]space[A.3.2.2d]space? - TBD |
Additional Information
- This cover page has Japanese license information only.
- If all the information is the same through all the numbered items, enter the numbers with one information. When there is no data available, skip the data and only print the serial numbers for which the values are present.
- If the license is withdrawn before the Start Date of the J-DSUR time frame, all the product information as well as the license information is not displayed.
- The overflow data is printed on the next page on the copy of this cover page that does not have any data other than the overflow data.
- For Ingredient Name, Indication, Study Development Phase, Amount, and Formulation, if source console values are not configured, fields on the paper form are left blank.
Parent topic: Clinical Study Periodic Safety Report (now J-DSUR)