- South Korea MFDS E2B(R3) Best Practices
- WHO codes for Products and Ingredients in Post-marketed Foreign Cases
- For Companies using WHO-DD C3 format
For Companies using WHO-DD C3 format
- For auto encoding of drugs during Case processing with WHO-DD C3 format, the dictionary is set in Console > Common Profile Switch > Case Form Configuration > Auto Encoding section.
- Set the same WHO-DD C3 dictionary in the newly introduced Common Profile Switch in Console > Common Profile Switch > E2B > Regional Drugs Dictionary.
Note:
This switch will have no impact on Case processing and that it is used only for MFDS E2B R3 reporting.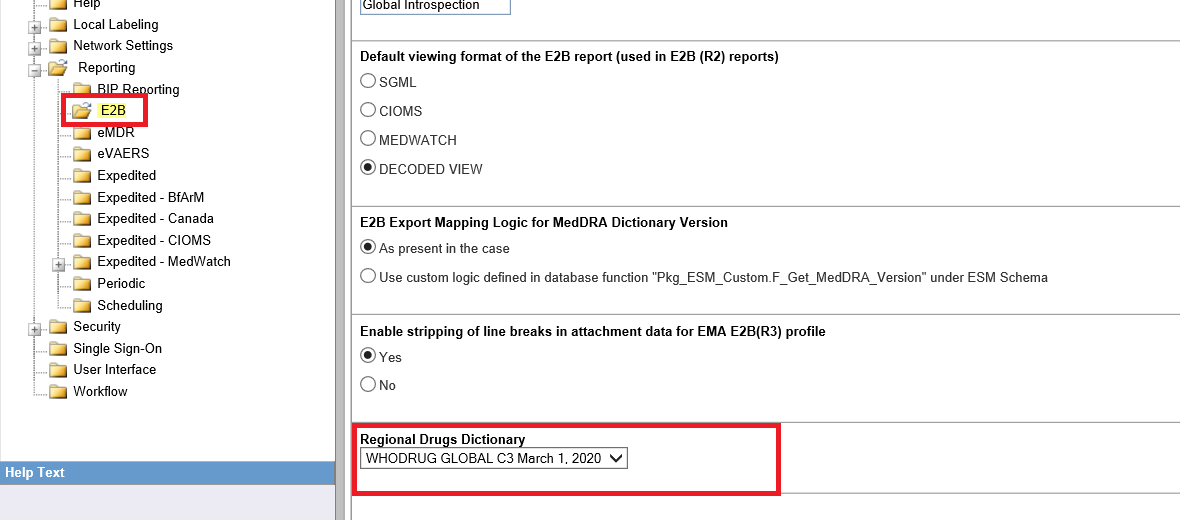
WHO Medicinal Product ID and WHO CAS Number is captured and transmitted as outlined in the table below.
For details, refer to the Business Rules defined in the E2B(R3) export mapping document.
Element ID Element Description Data Capture and Transmitted From D.8.r.1.KR.1a WHO-DD version Console > Common Profile Switch > E2B > Regional Drugs Dictionary D.8.r.1.KR.1b Medicinal Product ID (Patient Past drug Therapy)
Case Form > Patient > Other Relevant History > WHO Medicinal Product ID D.10.8.r.1.KR.1a WHO-DD version Console > Common Profile Switch > E2B > Regional Drugs Dictionary D.10.8.r.1.KR.1b Medicinal Product ID (Parent Past drug Therapy)
Case Form > Parent > Other Relevant History > WHO Medicinal Product ID G.k.2.1.KR.1a WHO-DD version Console > Common Profile Switch > E2B > Regional Drugs Dictionary G.k.2.1.KR.1b Medicinal Product ID (Suspect/Concomitant/Interacting Products)
Case Form > Product > WHO Medicinal Product ID G.k.2.3.r.1.KR.1a WHO-DD version Console > Common Profile Switch > E2B > Regional Drugs Dictionary G.k.2.3.r.1.KR.1b Substance ID (Ingredients of Suspect/Concomitant/Interacting Products)
WHO CAS Number is retrieved from WHO-DD C3 dictionary WHO_DRUG_C_SUBSTANCE table using
Case Form > Product > Substance Information > Substance Name
- For WHO drugsTo facilitate capturing of WHO Medicinal Product ID for Historical drugs, 2 Case Form fields are newly introduced, namely:
- Case Form > Patient > Other Relevant History > WHO Medicinal Product ID
- Case Form > Parent > Other Relevant History > WHO Medicinal Product ID
When drugs are coded during case processing with the WHO-DD C3 format, the WHO Medicinal Product ID is automatically populated from the WHO Drug browser.
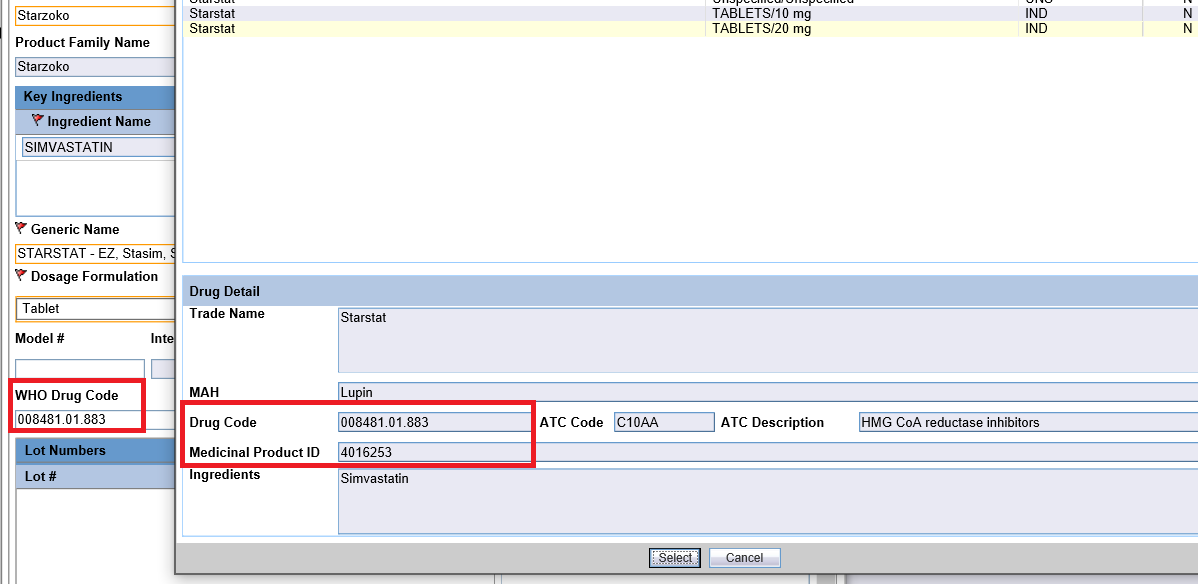
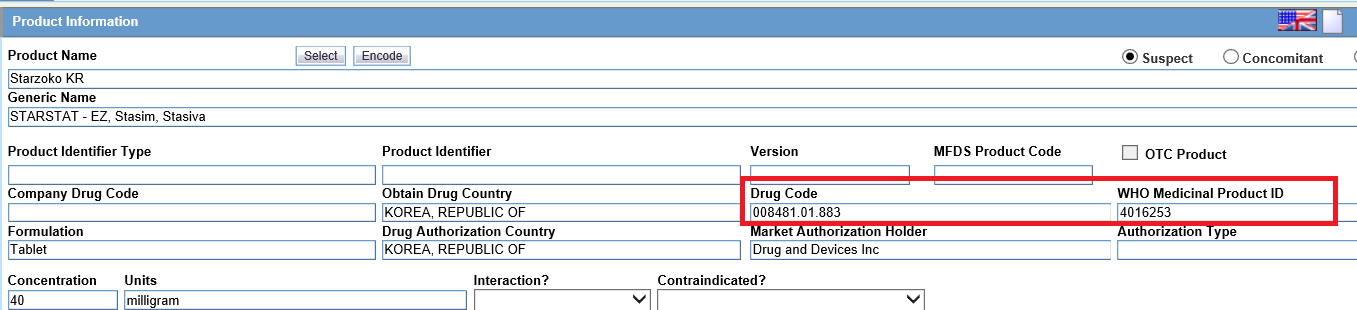
- For Company drugsIn Oracle Argus Safety Console, it is recommended to set the WHO Drug Code for company products. This will store the WHO Drug Code in LM_PRODUCT.DRL_ID and also the corresponding Medical Product ID in LM_PRODUCTS.MEDICINAL_PROD_ID.
When this Product is added in the case, the WHO Medicinal Product ID would be populated in Case Form > Product.
Note:
During E2B R2/R3 import, the WHO Medicinal Product ID will not be populated even if the incoming XML contains a WHO Drug product. This is because, with the elements available in the Page 15 of 28 XML file, it is not possible to determine the correct WHO MPID. Similarly, the system has not been populating WHO Medicinal Product ID in Case Form > Product during E2B R2/R3 import.