9 Approval/Acceptance Number (G.k.CN.4)
For post-marketed cases, G.k.CN.4 is transmitted with the license number configured in the Product and Licenses window based on the product and the license for which the report is generated.
The License number should be configured as required by NMPA in the Console, in the Product and License window, License# field.
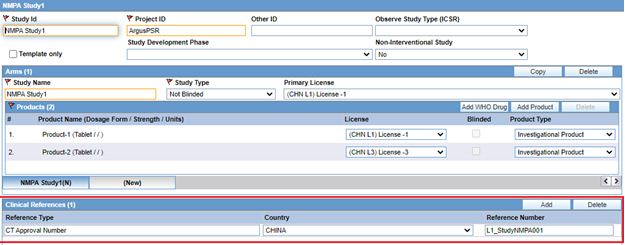
For clinical trial cases, G.k.CN.4 is transmitted with the application number configured on the Studies Configuration window in the Clinical References section based on the below logic. The Clinical References section should be configured so that G.k.CN.4 is transmitted as required by NMPA:
- Use Reference Type = CT Approval Number and Country = China
- Configure Reference Number in the format <License number>_<Application number>
For more details, refer to the business rule defined for G.k.CN.4 in the E2B(R3) export mapping document.