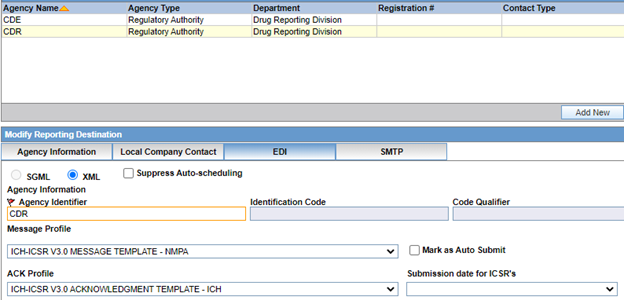
3 Reporting destinations setup
The Center for Drug Evaluation (CDE) and the Center for Drug Reevaluation (CDR) are two NMPA agencies.
CDE is responsible with submitting clinical trial reports and CDR is responsible with submitting post-marketed reports.
To achieve this, set up two different reporting destinations with agency identifiers in Argus.