Cumulative Summary Tabulation for Serious Unlisted Events
The Cumulative Summary Tabulations section is derived based on the Cumulative case series for Cumulative period event counts and Main case series for Current period event counts.
This tabulation includes only the S or UL Diagnosis events (relatedness of an event is not considered for this tabulation).
This table is organized first by HCP or Consumer, Product, and SOC, then by a comparison of events from current reporting with the cumulative reporting report.
Grouping
Cumulative Tabulations for Serious Unlisted (S or UL) cases report are grouped on the following options:
-
Grouping based on HCP or Consumer
-
Grouping based on Products within Product Selection
-
Grouping based on SOC
-
Grouping logic is as per requirements defined in Summary Tabulation for HCP Cases.
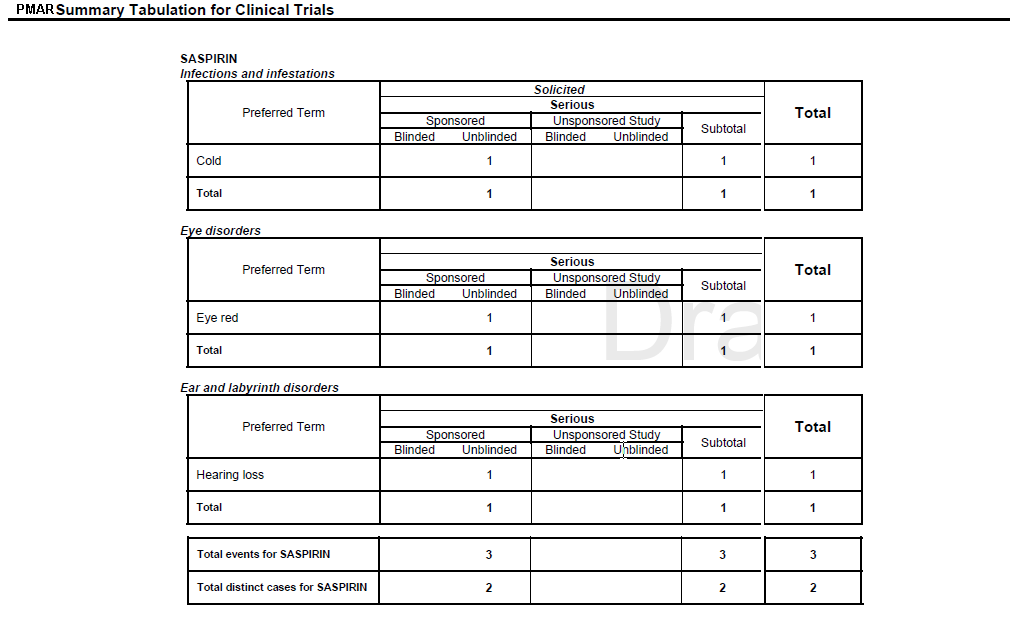
Clinical Trial Summary Tabulations
Table 7-6 Clinical Trial Summary Tabulations
| SI | Temp table reference |
|---|---|
|
Preferred Term |
LLT or PT from S or UL diagnosis events is printed based on the report parameter Print LLT instead of PT. LLT or PT is arranged in the alphabetical order within an SOC. |
|
Current Period |
The count of S or UL diagnosis events from the Main case series is computed against LLT or PT. Follow-up events from the Main case list are not considered for the Current Period. |
|
Cumulative Period |
The count of S or UL diagnosis events from the Cumulative case series is computed against LLT or PT. |
|
Total |
Summation of count of diagnosis event within SOC is printed under Current Period and Cumulative Period respectively. |
|
Overall total diagnosis for <Product> |
The count of diagnosis is printed against respective column at the end of Product grouping. |
|
Overall total of distinct cases for <Product> |
The count of Distinct Cases is printed against respective column at the end of Product grouping. |
Figure 7-4 Summary Tabulation for Clinical Trials Screen (Serious Unlisted Event)
Parent topic: Format for Clinical Trial Summary Tabulations