For Companies using WHO-DD B3 format
For drugs auto encoding during case processing with WHO-DD B3 format, the dictionary is set in Argus Console > System Configuration > System Management (Common Profile Switches) > Case Form Configuration > Auto Encoding section.
For MFDS E2B(R3) reporting, since it is mandatory to send the WHO Medicinal Product ID and the WHO CAS Number from C3 format, it is recommended to load the WHO-DD C3 dictionary into Oracle Argus Safety. Technically, Oracle Argus Safety supports loading multiple WHO dictionary versions and formats.
Note:
This switch shall not impact the Case processing and is used only for MFDS E2B(R3) reporting. Hence, companies can continue to code drugs with the WHO-DD B3 format as usual.MFDS E2B(R3) mapping logic is designed to automatically fetch the WHO Medicinal Product ID and the WHO CAS Number from the C3 dictionary set in the Regional Drugs Dictionary based on the WHO Drug Code in the Oracle Argus Safety Case Form.
This mapping is designed in discussion with WHO-UMC to fetch the match from C3 where Country = Unspecified, MAH = Unspecified, Formulation = Unspecified, Strength = Unspecified.
For example, for the ABAPEN drug coded using B3 format, Oracle Argus Safety mapping logic fetches the corresponding C3 format data, as in the image below:
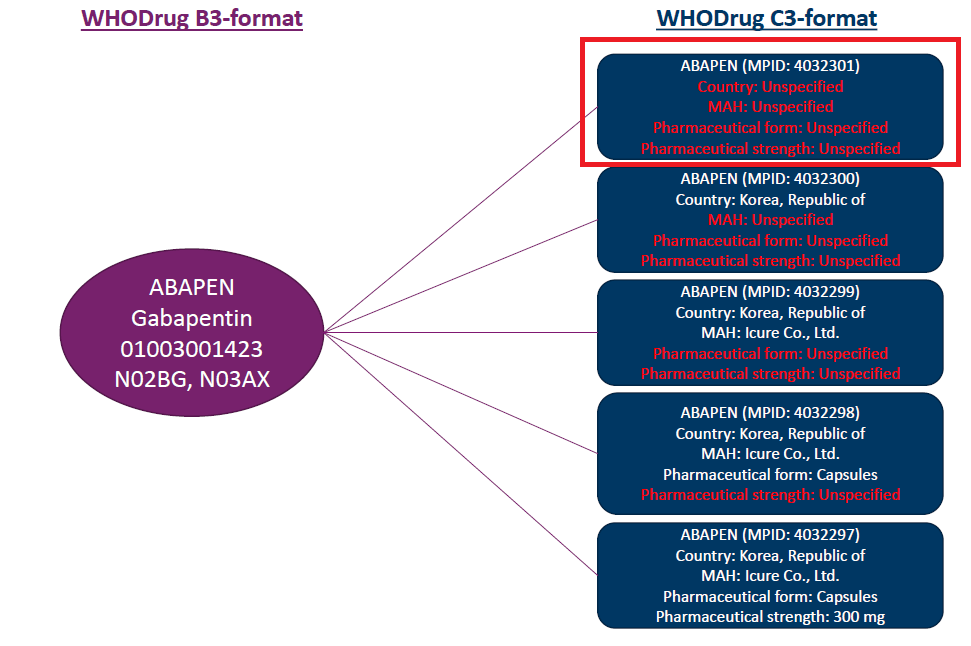
Note:
WHO-UMC does not provide official mapping between B format and C format. Oracle discussed this challenge with WHO-UMC, and they acknowledge the challenge. WHO-UMC are currently engaging with MFDS with the ultimate aim to create mappings between WHODrug B3 and WHODrug C3 format.Until official mapping between B format and C format is provided, it is recommended to use out-of-the-box MFDS E2B(R3) mapping logic in Oracle Argus Safety.
Note:
The customization of B3 to C3 mapping can be achieved by the customizing export mapping query for the below elements in Console > Interchange Mapping > MFDS profile:- PATIENTPASTDRUGTHERAPY
- PARENTPASTDRUGTHERAPY
- DRUG
- ACTIVESUBSTANCE
The pkg_mfds.sql file is unwrapped for this
purpose.
WHO Medicinal Product ID and WHO CAS Number are captured and transmitted as outlined in the table below.
For details, refer to the Business Rules defined in E2B(R3) export mapping document.
| Element ID | Element Description | Data Capture and Transmitted From |
|---|---|---|
| D.8.r.1.KR.1a | WHO-DD version | Console > Common Profile Switch > E2B > Regional Drugs Dictionary |
| D.8.r.1.KR.1b | Medicinal Product ID
(Patient Past drug Therapy) |
WHO Medicinal Product ID is retrieved from WHO-DD C3 dictionary WHO_DRUG_C_MASTER table using Drug code of the Patient past drug details |
| D.10.8.r.1.KR.1a | WHO-DD version | Console > Common Profile Switch > E2B > Regional Drugs Dictionary |
| D.10.8.r.1.KR.1b | Medicinal Product ID
(Parent Past drug Therapy) |
WHO Medicinal Product ID is retrieved from WHO-DD C3 dictionary WHO_DRUG_C_MASTER table using Drug code of the Parent past drug details |
| G.k.2.1.KR.1a | WHO-DD version | Console > Common Profile Switch > E2B > Regional Drugs Dictionary |
| G.k.2.1.KR.1b | Medicinal Product ID
(Suspect/Concomitant/Interacting Products) |
WHO Medicinal Product ID is retrieved from WHO-DD C3 dictionary WHO_DRUG_C_MASTER table using Case Form > Product > Drug Code |
| G.k.2.3.r.1.KR.1a | WHO-DD version | Console > Common Profile Switch > E2B > Regional Drugs Dictionary |
| G.k.2.3.r.1.KR.1b | Substance ID
(Ingredients of Suspect/Concomitant/Interacting Products) |
WHO CAS Number is retrieved from WHO-DD C3 dictionary WHO_DRUG_C_SUBSTANCE table using Case Form > Product > Substance Information > Substance Name |