Similar Incidents Dialog
Similar Incidents occurring with the same device type/variant of a manufacturer with same investigation finding (IMDRF investigation finding; Annex C) and the same medical device problem (IMDRF medical device problem).
Annex A) have to be reported to European Commission (EC). EC also allows usage of the in-house codes for Identification of similar incidents.
Oracle Argus Safety supports capture of the in-house codes for Medical Device Problem and Evaluation Result/Findings that may be used for computation of similar incidents, in addition to the IMDRF Codes.
The identification and computation of count of similar incidents is done outside the Oracle Argus Safety system and fields to capture the results are provided in Oracle Argus Safety.
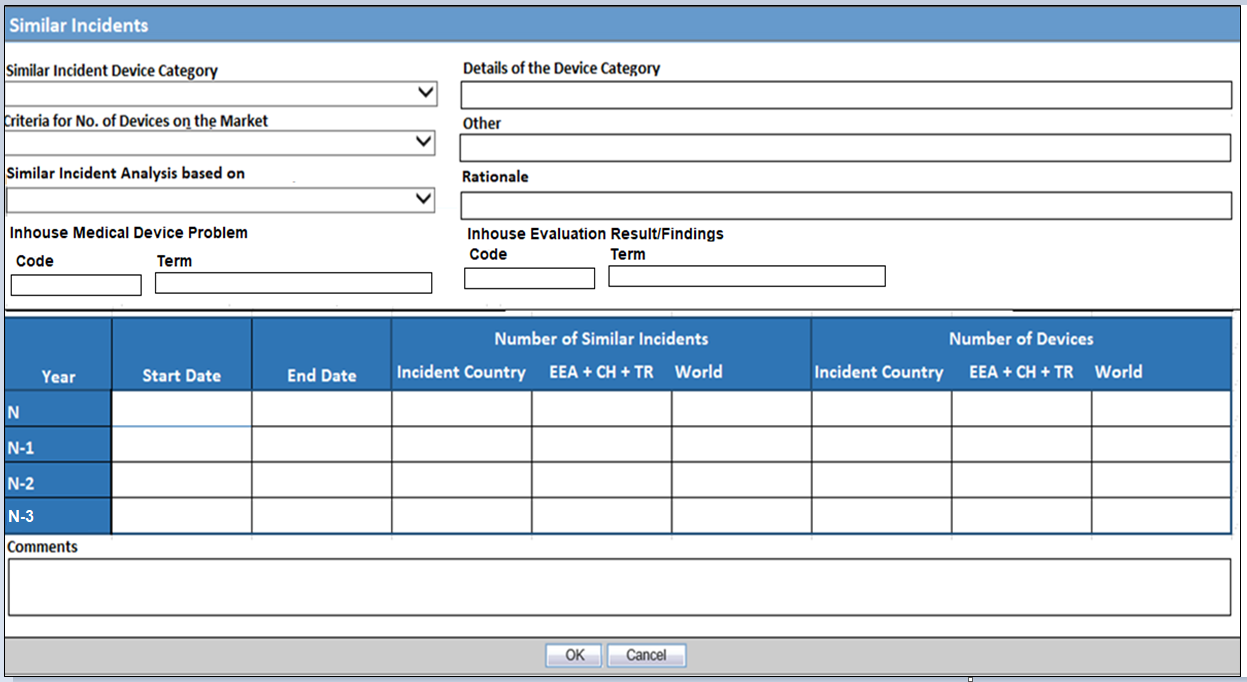
Similar Incident Information is Mandatory for MIR Combined Initial & Final and Final Reportable Incidents.
Parent topic: Case Processing and Reporting