What is an Annotated Study Book?
An Annotated Study Book is a form-by-form summary of the design of a study. Optionally, it includes a time and events schedule, a preview of each form, and selected annotations that list design details. Optionally, it includes a schedule of events, a preview of each form, and selected annotations that list design details. You can print an Annotated Study Book or save it to a PDF file and use it as a tool for reviewing the study design.
For details about how design information is displayed in an Annotated Study Book, see:
Schedule of Events table in the Annotated Study Book:
| Field | Description |
|---|---|
| Element |
If the study design uses study elements, they appear in the first row, and the study events in each study element are grouped under the study element columns. Note: The study element name for the special Screening and Enrollment visits is System. |
| Assessment |
Study events are listed in study workflow order, as they appear in the workflow editor for the study design or study element. The heading of each visit column shows the study event title, short title (in parentheses), and visit type (in square brackets). Visit types:
|
| CRF | Short title of each form, or blank if the ShortTitle property is not defined. |
| Visit Start Hours |
The visit start hour appears for study events. The visit start hour is a calculated value based on the scheduling specified for the study workflow. The start hour for the special Screening and Enrollment visits is zero. If the first visit after the Screening and Enrollment visits is not scheduled in the study workflow, its start hour is also zero. |
| Forms |
A number in each visit column where the form appears indicates the order in which the form occurs in the visit. Special form types are indicated by a code that follows the form order number:
|
| Key | Codes used for special types of forms and visits. |
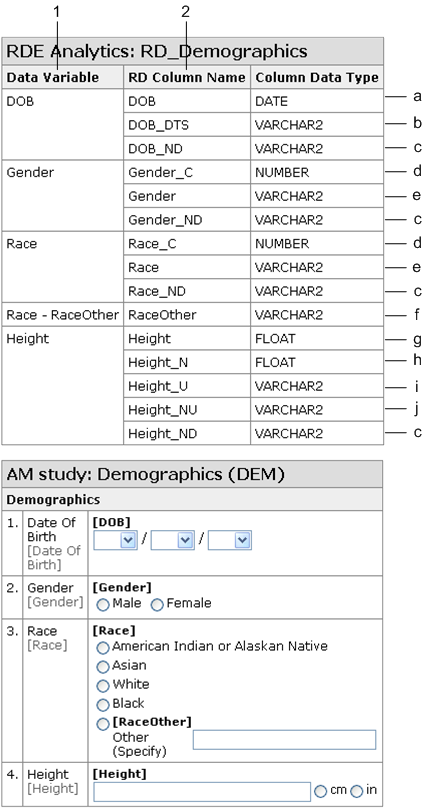
RDE Analytics tables
RDE Analytics tables are listings of the column names that are generated when study data is extracted to the Oracle InForm reporting database.
| Field | Description |
|---|---|
| Data Variable | Title of the item for which the column names are generated, or RefName if Show Object RefNames is selected in the Annotated Study Book Options dialog box. |
| RD Column Name |
Name of the column in the reporting database where the data will be inserted.
|
| Column Data Type | Data type of the column. |
| - |
Note: For each form, items that are outside of a section on a form and items that are in a nonrepeating section are included in a table. If a form contains a repeating section, each repeating section has its own table and the items in the repeating section are listed in the corresponding table for the repeating section. |
Example of an RDE Analytics table and its annotated form:
| Description | Illustration |
|---|---|
|
1—Study object RefName or Title. 2—Column name as it is generated in the InForm reporting database. a—Complete date time item. No suffix. b—Date time item with only date parts. c—Comment on item, reason why item was not done, or reason why item is incomplete. d—In an item with radio or checkbox controls or a drop-down list, the coded value for the selected control. e—In an item with radio or checkbox controls or a drop-down list, the label for the selected control. f—Child item in a compound or conditional item. RaceOther is a text item within the Race radio group. The column name contains the RefName of the parent item, a hyphen, and the RefName of the child item. g—Entered value of an item with units. h—Normalized value of an item with units. i—Unit symbol for an item with units. j—Normalized unit symbol for an item with units. |
 |
Parent topic: Reports and Annotated Study Book FAQs