Design environment
The CIS integrated design environment enables you to create metadata definitions of study components. The CIS synchronization functionality enables the transfer of study metadata and clinical data between the CIS servers and the InForm servers.
The following diagram illustrates the components of the CIS design environment.
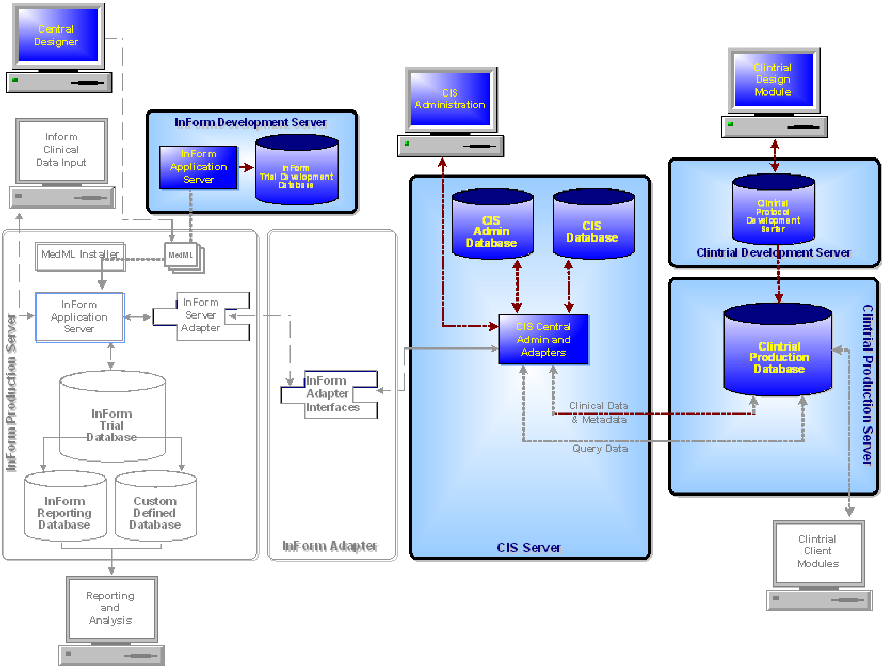
Component |
Purpose |
|---|---|
Central Designer software |
Design InForm study components and data mappings and send the Central Designer deployment package to an InForm study database. |
InForm application server |
Execute all InForm study activities. |
InForm study development database |
Store study metadata and clinical data for an InForm study that is being developed. |
CIS Administration application |
Configure the CIS environment and monitor synchronized protocol connections. |
CIS Admin database |
Store CIS administrative data. |
CIS database |
Retrieve, translate, and store Clintrial protocol metadata in MedML format. |
CIS Central Admin and adapters |
Perform CIS administrative activities and process transactions between the Clintrial software and the InForm software. |
Clintrial Design module |
Design a Clintrial protocol. |
Clintrial protocol development database |
Store and test new Clintrial protocol components. |
Clintrial production database |
Store protocol components, EDC study data, and paper-based study data for studies that are integrated with the InForm software. |



