Data transfer summary
The following diagram and table summarize the metadata and data transfers that occur during the development and production phases of an integrated study.
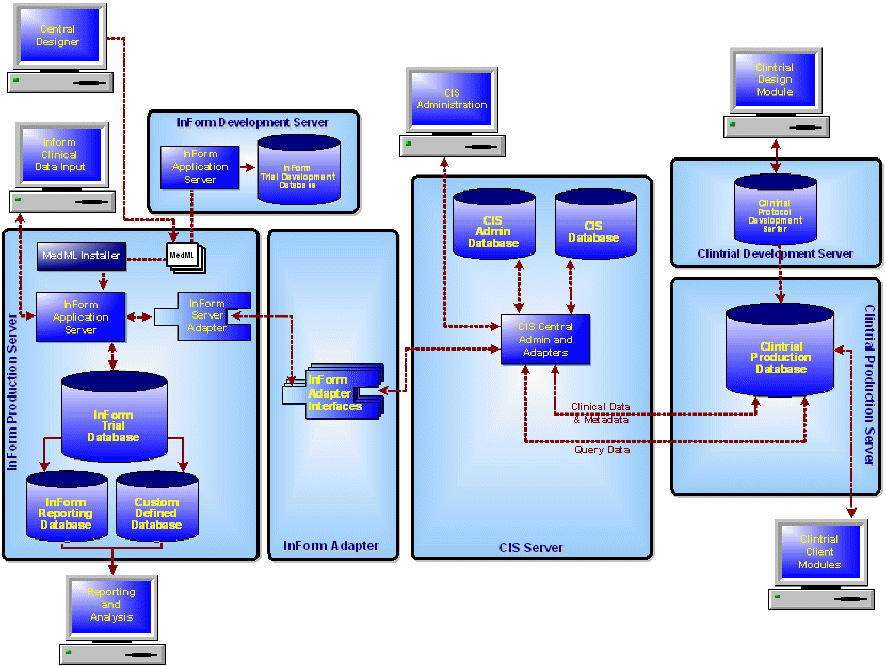
Data/Metadata |
From |
To |
Process |
|---|---|---|---|
Clintrial protocol metadata |
Clintrial software |
CIS Library |
When you register a Clintrial protocol in a CIS library, the CIS software translates the protocol to study MedML, generates Clintrial mappings, transfers the MedML, and stores the study metadata and mappings in the library. This occurs over an OLEDB connection. |
Central Designer deployment package |
Central Designer software |
InForm software |
When you deploy a study created in the Central Designer software to the InForm software, the Central Designer study components and workflow are translated to InForm study components. The conversion to InForm study components is based on both the data definition of each study component and the layout specified for each form or item in the Central Designer software. For more information, see the information about Central Designer and InForm study component correspondences in the Central Designer documentation. |
InForm study metadata and EDC clinical data |
InForm software |
Clintrial software via CIS software |
When you synchronize a study definition or clinical data from the InForm software to a Clintrial protocol, the CIS software:
|
Clintrial queries |
Clintrial software |
InForm software |
When a study is designed to validate EDC data with Clintrial rules and derivations, and the validation results in the generation of queries. |



