Report Parameters
When you run the report you can submit the following parameters:
- Investigator. Choose one from the List of Values or leave the wildcard (%) default to see results for all Investigators in this study.
- Site. Choose one from the List of Values or leave the wildcard (%) default to see results for all sites in this study.
- Patient. Choose one from the lists of values or leave the wildcard (%) default to see results for all patients in this study. Oracle Clinical will divide the report into sections by patient.
- DCM Short Name. Choose one from the list of values or leave the wildcard (%) default to see results for all DCMs in this study.
- DCM Subset Name. Choose one from the list of values or leave the wildcard (%) default to see results for all DCM Subsets in this study.
- Target Receipt Day Off Schedule. Optional; 29 if null.
- Start Date. The first date for which you want to see response data.
- End Date. The last date for which you want to see response data. If blank, the system uses the current date.
- Only from Earliest Visit Having a Missing DCM?. Enter Y or N for Yes or No.
- Discrepancy Query Status 1-3. You can query for up to three discrepancy review statuses. Optional; list of values available.
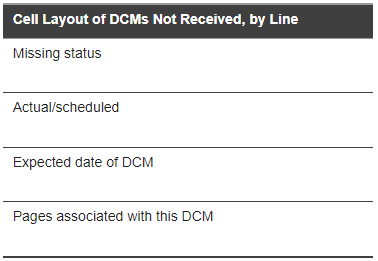
The following images illustrate a page from a typical DCM Detail Tracking Matrix report that documents two events. Visit 1 represents a Received DCM. Visit 2 represents a Missing DCM. For a key to the contents of the Visit column table cells, see Detail Tracking Matrix Table Cell Key: Received DCMs and Detail Tracking Matrix Table Cell Key: DCMs Not Received as shown below.
For a key to the status code abbreviations in those cells, see Data Entry Statuses, RDCM Validation Statuses, and Not-received DCM Statuses.
Figure 3-1 DCM Detail Tracking Matrix Report
Figure 3-2 Detail Tracking Matrix Table Cell Key: Received DCMs
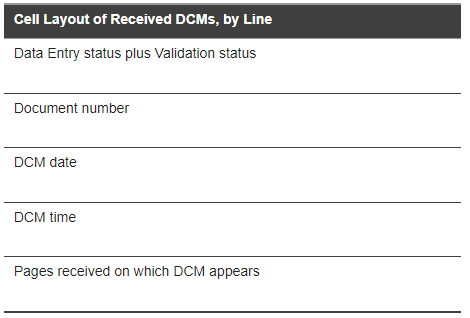
Figure 3-3 Detail Tracking Matrix Table Cell Key: DCMs Not Received
Parent topic: DCM Detail Tracking Matrix Report