How do I select hierarchy terms?
Adverse events and drugs can be associated with a dictionary, thesaurus, or other standardized terminology that organizes terms into a hierarchical structure. For example, adverse event terms may be associated with a version of the Medical Dictionary for Regulatory Activities (MedDRAFoot 1), and drugs may be associated with the WHO Anatomical Therapeutic Chemical (ATC) classification system. The hierarchical terms and their relationships are stored in a special account, then associated with variables in data configurations.
To select specific drug or event values for a variable, you can click the Select Available Values link to view and select values from an alphabetized list. If the drug or event variable has an associated hierarchy, another link appears so that you can browse through the hierarchy. For example, you can click Select MedDRA Terms to browse for Preferred Terms (PT) in the hierarchy. The variable must be set up in the data configuration and you must check the user preference, Enable Adverse Event Hierarchy Browser or Enable Drug Hierarchy Browser.
Note:
The name of the hierarchy associated with the variable appears as part of this link. For example, for a drug variable, Select ATC Terms appears. This help system uses Select <hierarchy> Terms as a general reference to this link.When you click Select <hierarchy> Terms, the Hierarchy Browser dialog box opens and enables you to browse the hierarchy and review, search for, and select terms at the appropriate level of the hierarchy.
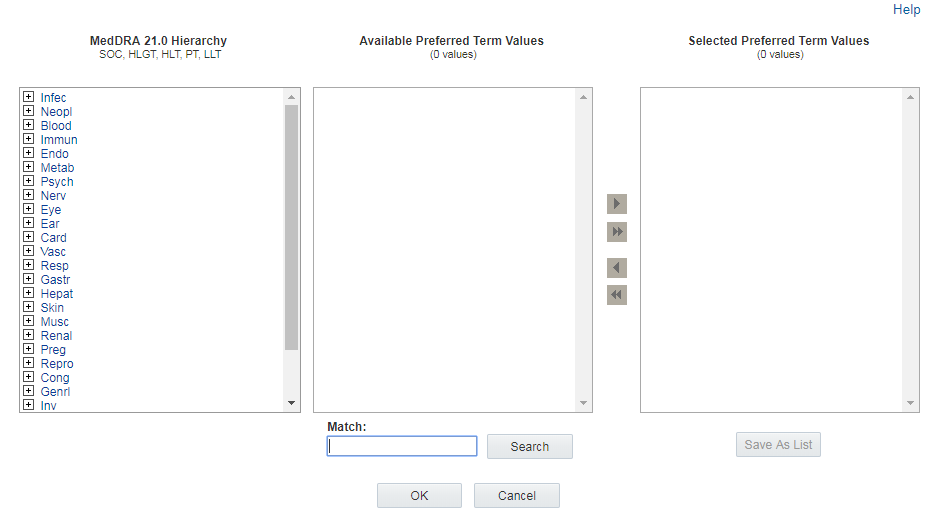
Browsing terms
The section on the left of the Hierarchy Browser lists the terms at the
highest, most general level of the hierarchy. For example, System Organ Class (SOC)
terms appear for the MedDRA hierarchy, and Level 1 terms appear for the ATC
hierarchy. To expand the list to show terms at the next level, click the ![]() icon next to a term. Repeat to expand the list to show terms at all levels
through the lowest, most specific level in the hierarchy. Click
icon next to a term. Repeat to expand the list to show terms at all levels
through the lowest, most specific level in the hierarchy. Click ![]() to collapse sections of the list.
to collapse sections of the list.
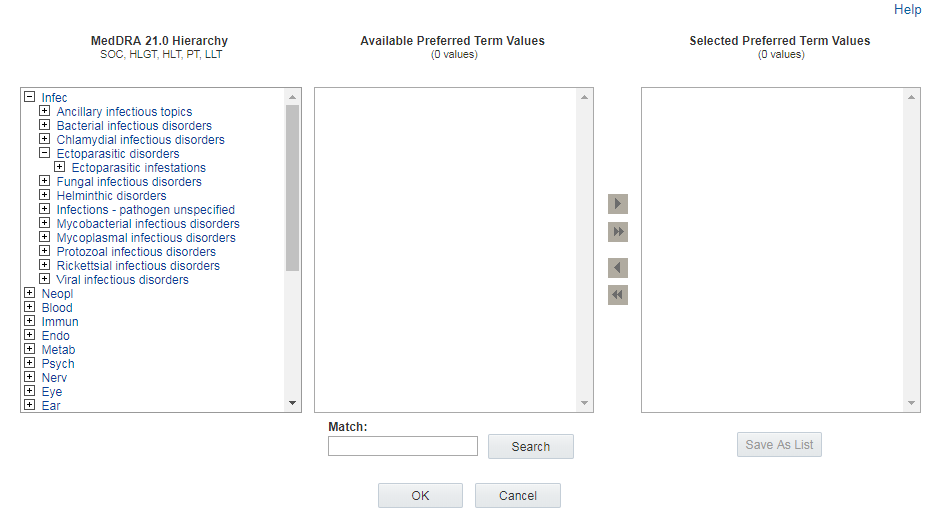
When you hover over terms in this list, an underline appears if you can click on a term to review and, potentially, select terms at the appropriate level of the hierarchy.
Reviewing terms
The Available <level> Values section in the center of the Hierarchy Browser lists terms at the level of the hierarchy that you are browsing. For example, if you are browsing for PTs, the Available <level> Values section lists all of the PTs in the hierarchy below the term you clicked. If you click on a Higher Level Group Term (HLGT), all of the PTs under that HLGT appear for review.
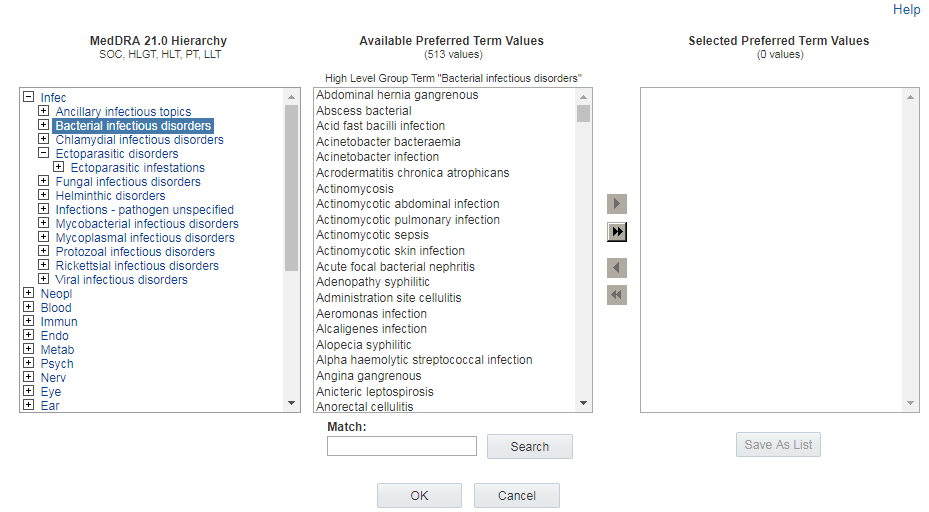
Note:
Not every term at every hierarchical level shown on the left can be clicked. For example, if you select MedDRA PTs, you cannot click on a lower level term (LLT) to review or select PTs above that LLT in the hierarchy.Searching for terms
Another way to find and review terms at the hierarchical level of interest is to enter text in the Match field at the bottom of the window and click Search. (The Match field is not case sensitive.) The list of Available <level> Values appears on the right and contains all terms that match your text at the appropriate hierarchical level. The hierarchy section on the left also refreshes; only terms at the top-most hierarchical level appear, and each term that has a matching term in its path appears in italics.
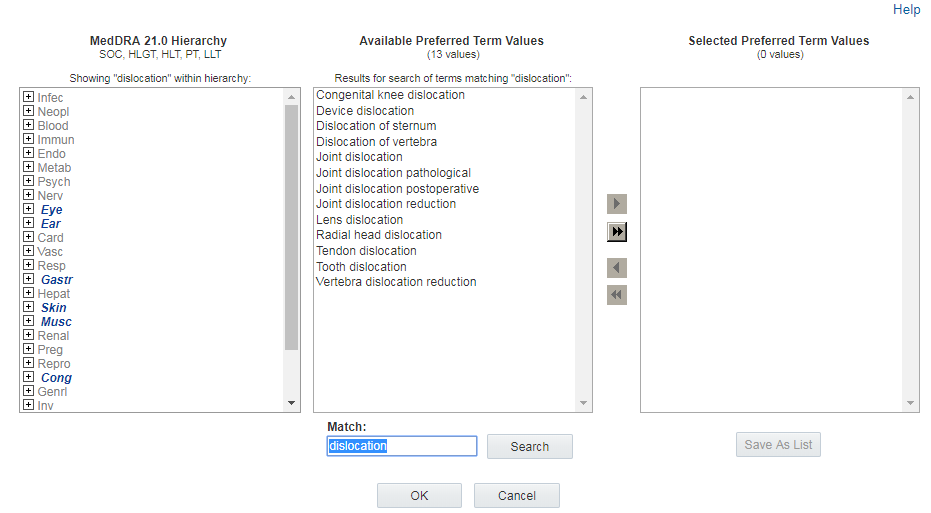
When you click ![]() next to an italicized term, the hierarchy expands all of the levels for
that term to show the complete path to the term that matches. Italics appear at
every level in the path to assist you in following the path to the matching term or
terms. Boldface highlights the actual match.
next to an italicized term, the hierarchy expands all of the levels for
that term to show the complete path to the term that matches. Italics appear at
every level in the path to assist you in following the path to the matching term or
terms. Boldface highlights the actual match.
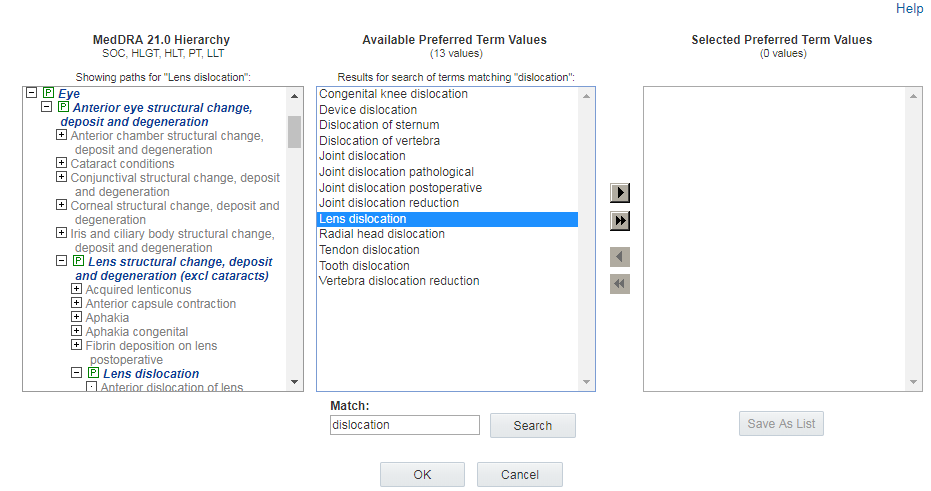
Alternatively, you can click a term in the Available <level> Values
list to expand the hierarchy section to show the primary path for that term. The
![]() icon indicates the primary path.
icon indicates the primary path.
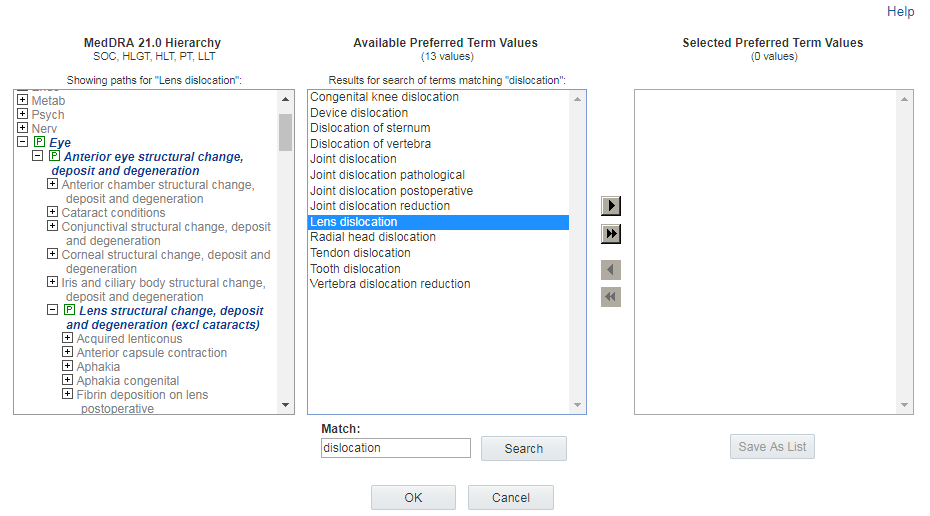
If the term also has a secondary path in the hierarchy, that path also
expands and marked by the symbol ![]() . You can scroll through the hierarchy to review any alternate paths for the
term.
. You can scroll through the hierarchy to review any alternate paths for the
term.
Note:
- When you click a term in the Available <level> Values list, its full name appears as pop-up text. This enables you to see the complete value in case the displayed value is cut off because of its length.
- Terms that appear in the hierarchy multiple times appear only once in the Available <level> Values list.
- The terms shown in this dialog box are from the special account that contains hierarchy data. As a result, custom terms do not appear in the hierarchy list, and cannot match entered search values.
Selecting terms
After you review terms in the Available <level> Values list, you can make selections. To select values, you highlight and move them as follows:
- To highlight a value, click it.
- To highlight multiple non-contiguous values, hold down the Ctrl key while clicking each value.
- To highlight multiple contiguous values, click a value, hold down the Shift key, and click another value. Values between and including those values are highlighted.
- To remove highlighting from a value, hold down the Ctrl key while clicking the selected value.
When your selections are complete, click OK. The window closes and you return to the task you were performing.
To create a saved list of terms for future use, click Save As List.
Parent topic: FAQs
Footnote Legend
Footnote 1: MedDRA® is a registered trademark of the International Federation of Pharmaceutical Manufacturers Associations (IFPMA). MedDRA® the Medical Dictionary for Regulatory Activities terminology is the international medical terminology developed under the auspices of the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH).