Configuration Screen
- Access the Argus Groups for Seiyakukyo report from Individual Report
Common Line List under the Console >
Groups > Menus section.
- This option appears under Reports > Periodic Reports right after J-DSUR menu option.
- It is marked enabled by default for Administrator Group.
- It is marked enabled by default during New Argus group creation.
- It is only displayed if Console > System Management > Enabled Modules > Japanese module is enabled.
- The following options are available:
- Include listed and unlisted events
- Include unlisted events only (default option)
- The Individual Report Common Line List menu is available
under the Argus Safety > Reports
> Periodic Reports menu.
- This option appears under Reports > Periodic Reports right after the J-DSUR menu option.
- This menu item is only available in Argus Safety when following
conditions are met:
Console > Access Management > Users > User Type of the current user is configured as ARGUS J USER.
Console > System Management > Enabled Modules > Japanese module is enabled.
Console > Access Management > Groups > Menus section for Argus Groups has Reports > Individual Report Common Line List marked Enabled for at least one user groups for the current user.
- Clicking on this menu in Oracle Argus Safety displays the existing Periodic Reports Library screen, which lists all the configured Seiyakukyo reports available in the system.
- The functionality of this screen remains the same as existing except for the following:
The page breadcrumb for the Periodic Reports Library screen for Seiyakukyo is displayed as: Reports > Periodic Reports > Serious, Unlisted AE Individual Case Communication Line List
The screen name for this screen is displayed as Serious, Unlisted AE Individual Case Communication Line List.
When you click the Open button for a submitted Seiyakukyo report whose configurable becomes non-editable, the following warning message is displayed: This configuration is not modifiable because the status of this Individual Case Communication Line List is Submitted
- The functionality of this screen remains the same as existing except for the following:
- Clicking New or Open buttons on
Periodic Report Library screen for Seiyakukyo reports
displays the Configuration screen for Seiyakukyo reports which has 5 tabs similar to
the J-DSUR Configuration screen with following names. The Page Title text is:
Individual Report Common Line List.
- Subject of Report
- Individual Report Common Line List
- Scheduling
- Security
- The Subject of the Report tab is the same as the existing Subject of the Report tab for J-DSUR.
- The Study Selection tab has the following
functionality:
Field Name Description Filter
This button is available adjacent to the Available Clinical Compound Numbers text box to allow filtering the data in the Available Clinical Compound Numbers multi-select list box. When you click this button, the data is refreshed in the Available Clinical Compound Numbers multi-select list box based on the search text specified by you in the textbox. Like search is performed based on the text specified by you. For example, If you have specified ABC as the search text, it performs case-insensitive text for %ABC%.The maximum allowed length of the Filter textbox is 70 characters.
Available Clinical Compound Numbers
It lists distinct Clinical Compound Numbers for all the licenses which are configured in the Console application.
Selected Clinical Compound Numbers
This list box is populated based on your selection and removal of Clinical Compound Numbers by using Add and Remove buttons from/to the Available Clinical Compounds list box.
This is a mandatory field. If it is not specified, and the OK button is clicked, an error message is displayed: Clinical Compound Number is not selected.
Available Study Products
This list box is auto-populated with Study ID, and Product Name.
It displays all products configured in Console > Study Configuration from all study arms including the WHO drugs and J drugs (from the English screen and Japan screen).
If a non-company drug is displayed in both English and Japan screens, then preference is given to the J drug name. That is, the Japanese values take preference to be displayed over the English values.
For company drugs, preference is given to the Product Name (J) and appears in the Study ID - Product Name format. Like, Study A - Product 1, Study A - J drug 1.
Selected Study Products
This list box populates products based on your selection in the Available Study Products list.
If you have neither selected Clinical Compound Number nor Study Product, then on click of OK, an error appears.
Add
This field allows you to move the selected item(s) from left hand list box to the right hand list box.
Add All
This field allows you to move all the items from the left hand list box to the right hand list box.
Remove All
This field allows you to move all the items from the right hand list box to the left hand list box.
Remove
This field allows you to move the selected item(s) from the right hand listbox to the left hand listbox.
Classifications for Clinical and Domestic Study Cases
It lists all the Case Classification (J) values from the Console code list. If J is not available, the corresponding English value is displayed.
You can select multiple values in this list box by using Ctrl + Click and Shift + Click combinations.
The first option in this list is always displayed as: All Case Classifications
These case classifications are used for fetching the case data for Clinical and Domestic study cases reporting in Seiyakukyo report.
Classifications for Foreign "Ichihen" Study Cases
It lists all the Case Classification (J) value from the Console code list. If J is not available, the corresponding English value is displayed.
You can select multiple values in this listbox by using Ctrl + Click and Shift + Click combinations.
The first option in this list is always displayed as: All Case Classifications
These case classifications are used for fetching the case data for Foreign Ichihen study cases reporting in Seiyakukyo report.
- Seiyakukyo Report tab has the following functionality:
# Field Name Description 1
Individual Report Common Line List
N/A
2
Common Configuration
N/A
3
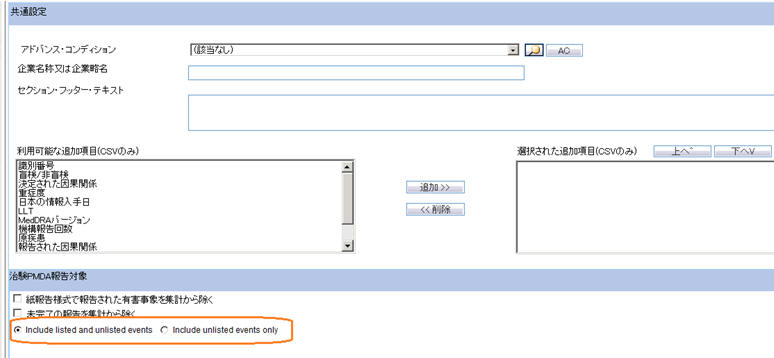
Company Name or Company abbreviation
You can specify the value of the Company Name to be printed in the second column of Seiyakukyo report using this field.
The maximum allowed length of this text box is 60 characters.
It allows English as well as Japanese characters.
4
Section Footer Text
You can specify a text to be printed as footer text at the end of each section in the report output using this field.
The maximum allowed length for this text box is 200 characters.
It allows English as well as Japanese characters.
5
Available Additional Columns (CSV Only)
This field lists all the available additional fields that can be added to the Seiyakukyo report CSV output. These are not applicable to the Word output.
The following fields are available in this list in the alphabetical order:
ACK Number
Blinded / Not Blinded
Determined Causality
Event Intensity
Japan Information Receipt Date
LLT
MedDRA Version
Number of times this report is submitted to PMDA
Primary Disease
Reported Causality
Report Submission Date
SOC
Study Name
Initial Receipt Date (J)
Aware date
Other References
Patient ID
Indication PT Term
Description as Reported
LLT Code
PT Code
SOC Code
6
Selected Additional Columns (CSV Only)
It lists all the selected additional fields that are added to the Seiyakukyo report CSV output. These are not applicable to the Word output.
You can move items between Available and Selected Additional Columns using the Add and Remove buttons.
While adding items, these items are listed in the same order in which they are added.
You can move items up and down using Up and Down buttons.
The Seiyakukyo report output includes these columns at the end in the same order as specified in this list.
7
Add
This field allows you to move the selected item(s) from the left hand list box to the right hand list box.
8
Remove
This field allows you to move the selected item(s) from the right hand listbox to the left hand listbox.
9
Up ^
This field allows you to move the selected item in the Selected Additional Columns, one position closer to top of the list.
10
Down V
This field allows you to move the selected item in the Selected Additional Columns, one position closer to the bottom of the list.
11
Clinical and Domestic Study Cases
N/A
12
Exclude events which were reported by paper report forms
If this check box is checked, all the events which are reported through paper reports are excluded.
This logic is applicable only for Clinical and Domestic Study cases for which events are reported based on Expedited/E2B reports.
It is unchecked by default.
13
Exclude Incompletion reports
If this check box is checked, all the events which are reported through Expedited/E2B reports where the value of J.6 (Mhlwadmicsrcompleteclass) = 1 (Incomplete) are excluded.
This logic is applicable only for Clinical and Domestic Study cases for which events are reported based on Expedited/E2B reports.
It is unchecked by default.
14
Include listed and unlisted events (new)
You can specify the criteria for printing the events under Regular and Domestic Study cases category using this field.
This radio button is selected by default if the "Include only serious unlisted event" check box was not selected for the already configured reports.
15
Include unlisted events only (new)
You can specify the criteria for printing the events under Regular and Domestic Study cases category using this field.
This radio button is selected by default:
- For all new reports.
- If the "Include only serious unlisted event" check box was selected for the already configured reports.
16
Foreign "Ichihen" Study Cases
N/A
17
Include foreign AE marked as "Not include for the report in Japan"
If this check box is checked, all the events in the cases which are marked as Not to be reported to Japan in the Case Form Events tab are also included.
This logic is applicable only for Foreign Ichihen study cases for which events are reported based on case data directly.
It is unchecked by default.
18
Append CIOMS reports
If this check box is checked, the CIOMS reports are created and added at the end of Seiyakukyo report output for all the cases which are printed under Foreign Ichihen Study Cases section. These CIOMS report are printed for the earliest Valid Japanese license for the first suspected product in the case. If the first suspected product does not have a Valid Japanese license, the next suspected product which satisfies this criterion is picked-up in the same order in which they are present in the case. Once the Seiyakukyo is generated as FINAL, these reports are listed in the Case under the Regulatory Reports tab in the similar manner as for English PSUR.These reports are added only in the PDF format (not in CSV), when the Seiyakukyo report is published.These reports are generated at the time of Seiyakukyo generation. The Generation Status dialog box displays the label Generating CIOMS reports for this task during the Seiyakukyo generation process. Although, generated at this stage, these CIOMS reports are visible to users after the Word format is published into PDF format. Even if you modify the Events/Cases printed in the application generated Word/CSV formats of Seiyakukyo report, the application still prints the CIOMS reports based on the application generated case list under Foreign Ichihen Study Cases section. It is unchecked by default.
19
DLP support
It displays 2 options to allow support of DLP for fetching case data for Foreign Ichihen study case: Use Current Case Version and Use DLP Case Version.
This radio button control is only displayed if DLP is enabled.
Based on the PSR/ J-DSUR configuration for DLP, the report is executed accordingly using the latest Case Revision or using the DLP Case Revision.
Use Current Case Version is always checked by default.
- The Scheduling tab is the same as the existing
Scheduling tab for J-DSUR, except for the following:
- The following date fields are not displayed as they are not
applicable to the Seiyakukyo reports. All the validation checks related to
these fields are also not applicable to the Seiyakukyo reports:
Assigned Date
Clinical Study Plan Submit Date
Clinical Trial for Partial Change Plan Submit Date
- The Start Date of first timeframe is editable as long as this timeframe is not submitted. However, the Start Date of second timeframes and onwards keep following the existing logic of being disabled and pre-populated as next day of the previous timeframe End Date.
- The following date fields are not displayed as they are not
applicable to the Seiyakukyo reports. All the validation checks related to
these fields are also not applicable to the Seiyakukyo reports:
- The Security tab is the same as the existing Scheduling tab for J-DSUR.