J-DSUR - Form 2
Summary: J-DSUR - Form 2 is the report of events that are serious (both Reported and Not Reported) during the specified investigational time period for Study drugs (including Study for partial change of the Marketed drugs).
- The past record is included in the report as cumulative Total Number of SOC and Terms.
- The number of the serious events which are either Reported or Not Reported to the PMDA within one investigational timeframe is the content for the report.
- Regardless of Incomplete or Complete report, all the events during the investigational timeframe are listed.
- If there are multiple events in one case, count each Reportable event separately. For example, if there are 2 same PTs available in the same case, the count is 1 SOC and 2 PTs.
- A case is included in the report for the current timeframe only if a significant Japanese follow-up is received for the case in the same timeframe. If there are no significant updates for the case, this case is not counted for the current period.
- If there are multiple follow-up updates (Significant or Non-significant) to a qualifying case in the same investigational time frame, count the events based on latest information on the events in the case and exclude the Deleted events during the timeframe. If the event is entered as Serious, but later in the same timeframe, this is changed as Non Serious, this event is not counted.
- The MedDRA version is the latest MedDRA J version at the time of report creation.
- The configured products that fall in DRUGCHARACTERIZATION = 1 (SUSPECTED) or 3 (INTERACTION) are collected. 2 (Concomitant) is not included.
- The date cut off for the data inclusion is based on the case's Japan first information Aware Date (in PMDA > General tab of the Case Form). If this is a follow-up case, the Aware (latest significant follow-up) Date.
- The Serious events must be Related events to be included in the list.
- An event which is included in the past J-DSUR report is included in the Cumulative Total section of the count in the report.
- For identifying the Japanese license, the license authorization country in the license configuration in Console is used. For the count of the foreign data, all configured products that have the same ingredients (regardless of the existence of Japanese license in this family) is considered as the target.
- When the End Date is set as past date, any updates/new data that meet above condition in between the End Date and the current date are not included in this line listings. The data is the latest in the given timeframe, and not between the investigational timeframe Start Date and the Report Generation Date. This is achieved when you have enabled DLP and has configured the Use DLP Case Version radio option for J-DSUR configuration.
- If there is 0 count on any counting numbering sections of the Line Listing, - is printed.
- From 2nd and subsequent pages, the table header rows are repeated at the top of the each page.
Basic Data Retrieval Rule:
Cumulative Total:
- In J-DSUR, the Cumulative Total is the count of Serious AEs that are collected between the Assigned Date and the end of the current investigation timeframe.
Investigation Time Frame
The Serious AEs from the cases collected in this investigation timeframe from among the cases retrieved by Cumulative Total.
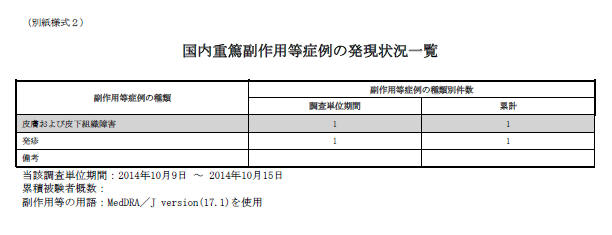
Domestic Serious AE, etc. Case Occurrence Status Line Listing
| Field Name | Description |
|---|---|
|
Serious AE, etc. Case Occurrence Status Line Listing |
This field represents the title of the paper form. |
|
Attached paper form |
This field represents the header of the paper form. |
|
Information Source |
This field represents the title of the row. |
|
Clinical Study |
This field represents the title of the column. This column has data only from the report source: Clinical study and Incident Country = Japan. It only considers the cases which contain the studies configured as Selected Studies in the Product Selection tab. |
|
Investigation Time Frame |
This field represents the title of the row. |
|
This investigation period |
This field represents the title of the column. This column collects count information in this investigation period only. |
|
Cumulative Total |
This field represents the title of the column. This column collects total number of count information in the entire reporting period starting from the first Assigned Date to the end of current investigation period. If the configuration is set (checked for Exclude the event count from the cumulative total if the event doesn't meet the condition), events that were reported in the past but as the condition has been modified in the current timeframe they no longer meet the condition, are removed. |
|
This investigation period |
This field represents the title of the column. This column collects count information in this investigation period only. |
|
Cumulative Total |
This field represents the title of the column. This column collects the total number of count information in the entire reporting period starting from the first Assigned Date to the end of current investigation period. |
|
This investigation period |
This field represents the title of the column. This column collects count information in this investigation period only. |
|
Cumulative Total |
This field represents the title of the column. This column collects the total number of count information in the entire reporting period starting from the first Assigned Date to the end of current investigation period. |
|
Number of subjects |
This information is captured from the J-DSUR tab. |
|
AE, etc Case classification |
This field represents the title of the column. |
|
AE, etc. Case Count |
This field represents the title of the column. |
|
SOC name in Japanese |
The SOC count is the count of cases (not AEs). The number is collected for this inv. Period and the total of entire J-DSUR time. |
|
PT names |
All the serious AEs are counted and entered for this inv. Period and entire J-DSUR time. |
|
This investigation time frame: YYYY/MM/DD |
This investigation timeframe is entered using the Japanese date format. |
|
Terms for AE: MedDRA/J version ( ) was used. |
The used MedDRA/J version must be entered in the brackets. |
Parent topic: Clinical Study Periodic Safety Report (now J-DSUR)