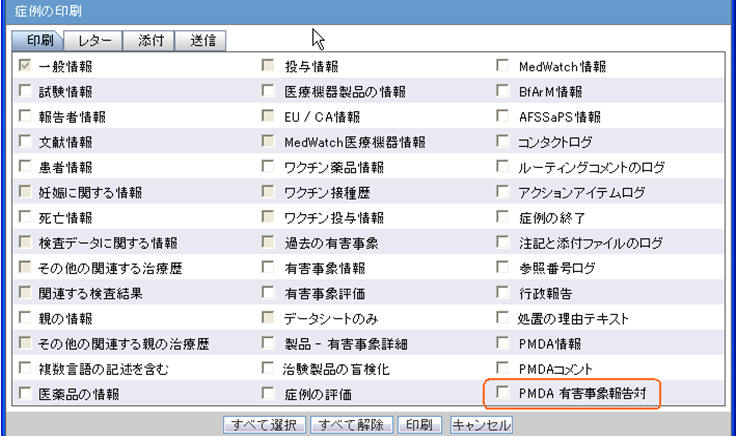
PMDA Event Reportability
- "PMDA Event Reportability" has been added to the Print Case dialog which opens up from Case Actions > Print > Case Form (Ctrl+Alt+P) in the location specified in the mockup screen below.
- This option is displayed only for Argus J users.
- This option is enabled for the Users belonging to at least one User Group having Modify/View access to Console > Access Management > Groups > Case Form > Case Analysis-PMDA option.
- Select All and Deselect All buttons update the check box for this section as well.
- On marking this option in Print Case dialog, the "PMDA Event Reportability" section is printed in the Case Form report after PMDA Comments section.
- All the fields of the PMDA Event Reportability section in Case Form Report print data as per the version of the case opened.
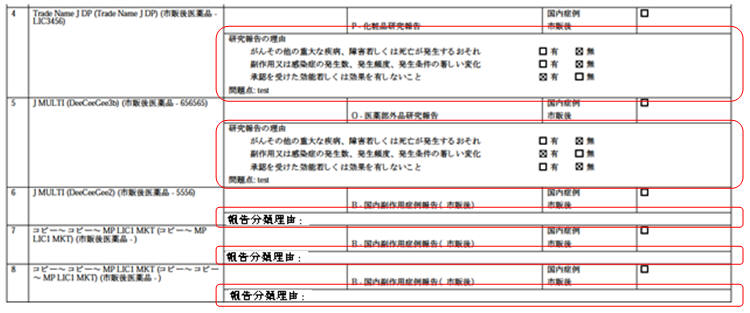
- A green dot symbol is displayed right after the Reporting Category dropdown value in
Case Form PMDA Comments sub tab for each product license as
soon as a reporting category is selected for that product license.
- Research Reporting Category:
If the user selects a Research Reporting Category (E, F, L, M, O and P), then the application retains the existing functionality of automatically opening the existing Research Reporting Category Justification dialog.
All existing functionality related to Research Reporting Category Justification dialog is retained as is.
- Other Reporting Category:
If the user selects any other Reporting Category, then the standard Justification dialog opens up automatically.
Manual clicking on the green dot symbol also opens up the standard Justification dialog to capture the final assessment text in Japanese for that product license as displayed below.
The dialog displays the list of pre-configured justification text from Justification code list for this field. By default, Not Specified is available in Justification code list for this field.
The justification dialog is displayed in Japanese to capture final assessment text in Japanese with max length of 20000 characters.
The title of this justification dialog is displayed as Assessment Justification.
The justification text is optional and the green dot is always displayed for non-research reporting categories even if no justification text is specified so that user can click on it and provide it at any point of time later.
- Data fields from Justification dialogs for both research as
well as non-research categories are printed in Case Form Print along with
PMDA General tab > Product License section as shown below:
- Research Reporting Category:
-
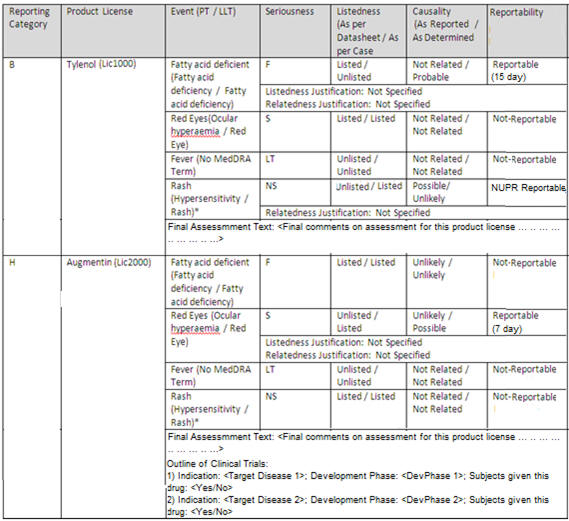
The PM DA section contains the following columns:
- Reporting Category:
This column prints the list of distinct Reporting Categories for the Japanese Product License as selected in PMDA tab in an alphabetical order.
Reporting Categories which are not mentioned in PMDA-General tab are not printed in PMDA Event Reportability section.
- Product License:
This column prints the Japanese Product Licenses as listed in the PMDA General tab against the associated reporting categories listed in the first column in the same order, as listed in the PMDA General tab.
The product licenses which do not have a reporting category assigned to them are not listed in this section.
- Assessment Reason:
This is printed at Product License level at the end of all the event rows.
It is printed in the format - "
 : <Value>".
: <Value>".
The value is printed as specified on Case Form > PMDA Comments sub tab > Product License section > Reporting Category > Research Reporting Justification dialog > "Reason for subject of the Research Report - Problems" for research reporting categories (E, F, L, M, O and P) and regular Justification dialog for all other reporting categories. for the current product license.
If no value is available to print, then only header text is printed.
- Outline of Clinical Trials:
This is printed at Product License level at the end of all the event rows.
This is printed only for Investigational Reporting categories (H, I, J, K, L, M and N). It is left blank for other reporting categories as this information is not printed in PMDA Expedited Reports for other reporting categories.
It is printed based on data from all the studies configured in Console with matching clinical compound number as for the current product license configuration.
Latest study configuration data from Console would be used to print these fields for the case revision data being printed.
It is printed in following format:
- Event ()
This column prints all the events entered in the case in the format: <Description as reported in Japanese (Event PT (J) / LLT (J))> as displayed on the screen mockup below.
If description as reported in Japanese is missing, then the following is printed in the Event column:
<Desc. as reported in English>
All these events are repeated against all the product licenses, listed in the same order as in the Case Form > Event Assessment tab
An Asterisk(*) is printed in this column for the events that are marked as "Do not include in Reports" in the below format:
<Description as reported in J> (PT (J) / LLT (J))*
A footnote "Event that are excluded from reportability" is printed at the end of Event Reportability section of Case Form Report if the case has events marked with "Do not include in Reports" in the Case Form Events tab.
The text "No MedDRA Term" is printed for events which are not encoded in the below format:
<Description as reported (No MedDRA Term)>
- Seriousness
If the Event is "Fatal", then '"F" is printed against the Event.
Else if the Event is "Life Threatening", then "LT" is printed against the Event.
Else if the Event is neither "Fatal" nor "Life threatening", but has any other Seriousness criteria marked, '"S" is printed against the Event.
Else "NS" is printed against the Event.
However, if the Event is neither "Fatal" nor "Life threatening" and the Console Common Profile Switch under Argus J > E2B > "Seriousness criteria in Event Reportability Matrix" is configured to "Case Level Seriousness", then the value for this column is printed same for all events based on Case Form > Analysis tab > Case Seriousness field.
- Listedness
This column prints the Listedness of the event as per datasheet followed by listedness as determined in the case. It is assessed against the same datasheet version that is used in Case Form Event Assessment tab.
This column prints 'Unlisted' for Unlisted events and 'Listed' for Listed events as per the listedness specified in Case Form Event Assessment tab.
This column prints 'Unknown for events with Listedness set to 'Unknown'.
This column prints 'Data not entered' for events without listedness entered.
While determining the listedness as per datasheet, only latest datasheet from Console would be executed against the case revision data being printed.
- Causality (As Reported / As Determined)
This column prints the Causality As reported followed by causality as determined.
This column prints the causality terms in Japanese as per the Event Assessment tab for "As Reported", and "As Determined".
This column prints 'Data not entered' for events without relatedness entered.
- Listedness Justification
If Listedness Justification (J) is available for the Product License - Event, then it is printed in a row below Seriousness, Listedness, and Causality columns.
If Listedness Justification (J) is not available, then Listedness Justification entered in English is printed.
Otherwise, Listedness Justification row is not printed.
This value is printed in the format as below:
<Justification Value>
- Causality Justification
If Causality Justification (J) is available for the Product - Event, then it is printed in a row below Seriousness, Listedness, Causality columns.
Otherwise, If Causality justification (J) is not available, then Causality justification entered in English is printed.
Otherwise Causality justification row is not printed.
This value is printed in the format as below:
<Justification Value>
If both Listedness Justification and Causality Justification are available, then these are printed in the same row but in separate lines.
- Reportability
This column prints 'Reportable' or 'Non-Reportable' based on the final reportability of the event as per the existing logic in the Event Reportability matrix.
For reporting category A and B only, if the event is not reportable as per PMDA Event Reportability matrix criteria then it is assessed if it is reportable for Non-Serious Unlisted Periodic Report (PSR Form 7-2) as per the following criteria. If it is reportable in PSR Form 7-2, then 'NUPR Reportable' is printed, Otherwsise, 'Non-Reportable' is printed.
Event is not serious as per Case Form Events tab
Event is related (either of As Reported or As Determined Casuality is reportable) for the current product as per Case Form Event Assessment tab.
Event is unlisted for the current product license as per Case Form Event Assessment tab
Event is not marked as Infection event as per Case Form Events tab
For all the events which are "Reportable", the same cell also prints the event reporting timeframe for which this event qualifies to be reported.
This value is printed right under the "Reportable" text in format - "(<xx>?)" e.g. "(15?)" (15 days)
This value is derived for each event based on the report scheduling rules configured in Console for the current license and current reporting category. The shortest timeframe from the matching rule is printed.
Latest reporting rules from Console would be executed against the case revision data being printed.
As reporting rules are executed against the case data in the database, hence any unsaved changes in the case would not be accounted while matching the reporting rules.
If no matching rules are found, then nothing is printed for event reporting timeframe.
- Reporting Category:
- If the Product, Event or Justification data do not fit in their respective columns, the text wraps within its own cell of the table.
- This information as displayed below is printed below a header row with section label:
"PMDA Event Reportability" (similar to header row for other case form section).
- If no data is present for this section then, it prints "No information present" in the header row itself (same as for other case form sections).
Figure 2-1 Case Form - PMDA Event Reportability section of Case Form Report
Parent topic: Case Actions