DSUR Tabulation Format
The report prints the count for medicinal products in the first column, followed by Blinded, Active Comparator, Placebo, and No Study Drug Given.
If the event count is 0 for Medicinal Product, Blinded, Active Comparator and Placebo columns for a given SOC, the system does not print that SOC in the report.
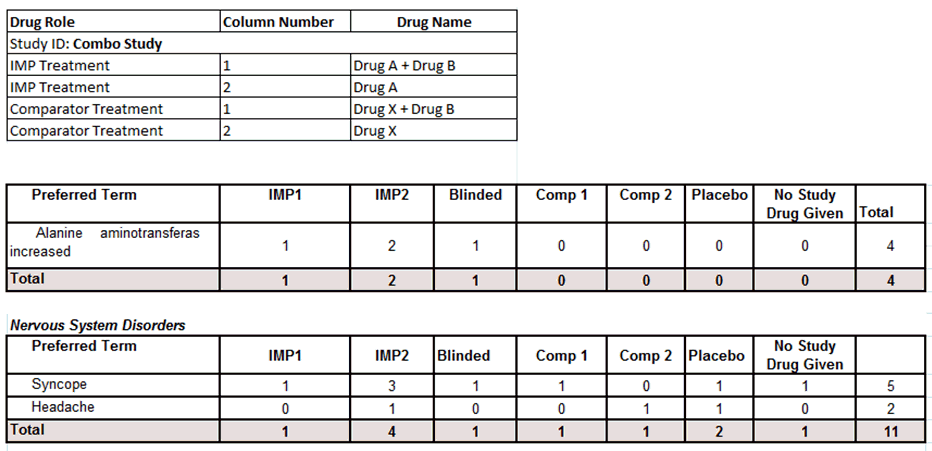
The DSUR tabulation prints the short name for investigation drugs and comparator drugs. For investigational drugs, the system prints SD1, SD2, SD3, and so on, based on the number of study drugs that are part of the investigational product column count. For comparator drugs, the system prints the short name as Comp1, Comp2, and so on, based on the number of comparator drugs that are part of the Active Comparator column count.
The system provides a table of information for all tabulations for detailing the study drugs and comparator drugs names used in the tabulation corresponding to each short name. This table has the following format and contains the following fields:
Table 6-5 Drug Roles and their Descriptions
| Drug Role | Description |
|---|---|
|
Study ID |
Name of the study ID used in the tabulation grouping. |
|
Study Drugs |
The system prints the column number of the study drugs used in the tabulation and its actual name in the Drug Name column. |
|
Comparators |
The system prints the column number of each comparator drug used in the tabulation and its actual name in the Drug Name column. |
Note:
The system displays all SUSAR events with an asterisk *. The SUSAR event term (PT or LLT) appears with an asterisk to the right of the term. For example, SEPTICEMIA*.
The system displays all special interest events with a superscript †. For example, OEDEMA†. You can configure the list of special interest events for a report by using the Oracle Argus Safety CTPR configuration screen.
Drugs configured as Placebo or No Study Drug Given are not part of the summary table.
Data Example
- Drug A – IMP
- Drug B – Additional Study Drug
- Drug X – Comparator
Drugs configured as Placebo or No Study Drug Given are not part of the summary table.
Parent topic: Summary Tabulations of Serious Adverse Events