Grouping
Summary Tabulation for solicited (clinical) cases report is grouped based on the following options:
- Grouping based on products within Product Selection
- Grouping based on SOC
| Column headers | Description |
|---|---|
|
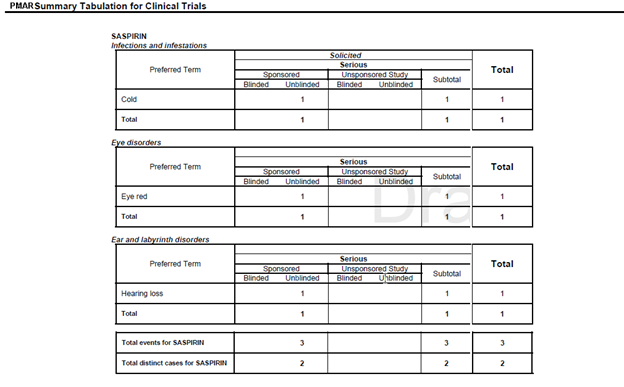
Preferred Term |
LLT or PT is printed based on the report parameter Print LLT instead of PT. Application prints diagnosis if the report parameter Use Only Diagnosis Events is Y. If this parameter is N, the Diagnosis and Symptoms are printed. LLT or PT is arranged in the alphabetical order within a SOC. |
|
Solicited Serious-Sponsored-Blinded |
The count of serious events from blinded cases, having the report type value as Non-Sponsored Study for REPTYEPGRP and as Solicited for CASETYPETEXT, is printed in this column. |
|
Serious-Sponsored-Unblinded |
The count of serious events from unblinded cases, having the report type value as Non-Sponsored Study for REPTYEPGRP and as Solicited for CASETYPETEXT, is printed in this column. |
|
Serious-Non-Sponsored-Blinded |
The count of serious events from blinded cases, having the report type value as Non-Sponsored Study for REPTYEPGRP and as Solicited for CASETYPETEXT, is printed in this column. |
|
Serious-Non-Sponsored-Unblinded |
The count of serious events from unblinded cases, having the report type value as Non-Sponsored Study for REPTYEPGRP and as Solicited for CASETYPETEXT, is printed in this column. |
|
Sub-total |
It is computed by summation of serious sponsored and unsponsored events from Solicited cases. |
|
Total events for <SOC> |
The count of events are printed against the respective column at the end of SOC grouping. |
|
Total events for <Product> |
The count of events are printed against the respective column at the end of the Product grouping. |
|
Total of distinct cases for <Product> |
The count of distinct cases are printed against the respective column at the end of Product grouping. |
Parent topic: Summary Tabulation for clinical cases