PMAR Summary Tabulation
-
HCP and Non-HCP cases are tabulated in this section of the report. The table is organized first by Initial or Follow-up, Product and SOC, then by Solicited or Non Solicited, Seriousness, Study Type (Sponsored versus Unsponsored Study).
-
Only related events (related to company drug that is specified in product selection) are considered for this tabulation for solicited cases. Relatedness information is not considered for Non-Solicited cases.
-
If the report parameter Exclude non serious cases from summary tabulations is set to Y, the system still prints the Grouping and Counts based on Non-Serious events which are part of serious cases. Only Non-serious cases and corresponding events are ignored based on parameter value of Y.
Grouping
Summary Tabulations for HCP cases report are grouped on the following options:
Table 7-3 HCP Case Grouping
| Group or sort options | Description |
|---|---|
|
Grouping based on Initial or Follow-up |
Cases are listed under Initial or Follow-up based on temp table logic. If the report parameter Include Follow-up cases from Summary Tabulation is set to N, then summary tabulations are not printed for follow-up cases. |
|
Grouping based on Products within Product Selection |
Cases with the company suspect products matching with the products selected in the report configuration are grouped. Drug names that appear in the group header are arranged in ascending order. If there are no cases for a drug that is specified in the drug list, then this drug name does not appear as a header. If a case includes multiple company suspect drugs that are in the drug list, the case is presented in each drug section; the case appears multiple times in the report. |
|
Grouping based on SOC |
Cases are grouped by the SOC of the Primary Event. SOC group headers are displayed in the Internationally agreed order as specified in the codelist SOC_DISPLAY_ORDER. If the Report parameter List cases in the Line Listing under SOC for each diagnosis is Y, the case is listed multiple times under different SOCs. If this parameter is set to N, the case is printed under the SOC of the primary event, and a reference to this information is printed for the SOCs of other events. |
HCP Summary Tabulation Columns
Table 7-4 HCP Summary Tabulation Columns
| Column headers | Description |
|---|---|
|
Preferred Term |
LLT or PT is printed based on the report parameter Print LLT instead of PT. System prints diagnosis if the report parameter Use Only Diagnosis Events is Y. If this parameter is N, then diagnosis as well as symptoms are printed. LLT or PT is arranged in alphabetical order within a SOC. |
|
Solicited |
Solicited cases are clinical trial cases having at least one event related to the drug either the company or reporter causality flag is Y. |
|
Serious-Sponsored |
The count of Serious Events with report type group as Sponsored Study, and case type text as Solicited is printed in this column. |
|
Serious-Non-Sponsored |
The count of Serious Events with report type group as Non-Sponsored Study, and case type text as Solicited is printed in this column. |
|
Sub-total |
The sub-total is computed by summing-up Serious sponsored and unsponsored events from Solicited cases. |
|
Non-Solicited |
Non-Solicited cases are Non-Clinical trial cases having at least one event related to the drug. Either the company or reporter causality flag is Y. |
|
Serious-Direct to MAH |
The count of Serious Events with report type group as Direct to MAH, and case type text as Non-solicited is printed in this column. |
|
Serious-HA |
The count of Serious Events with report type group as HA, and case type text as Non-solicited is printed in this column. |
|
Serious-Stimulated |
The count of Serious Events with report type group as Stimulated, and case type text as Non-solicited is printed in this column. |
|
Serious-Literature |
The count of Serious Events with report type group as Literature, and case type text as Non-solicited is printed in this column. |
|
Sub-total |
The sub-total is computed by summing-up Serious events from Non-Solicited cases. |
|
Non-Serious -Direct to MAH |
The count of Non-Serious Events with report type group as Direct to MAH, and case type text as Non-solicited is printed in this column. |
|
Non-Serious-HA |
The count of Non-Serious Events with report type group as HA, and case type text as Non-solicited is printed in this column. |
|
Non-Serious-Stimulated |
The count of Non-Serious Events with report type group as Stimulated, and case type text as Non-solicited is printed in this column. |
|
Non-Serious-Literature |
The count of Non-Serious Events with report type group as Literature, and case type text as Non-solicited is printed in this column. |
|
Sub-total |
The sub-total is computed by summing-up Non-Serious events from Non-Solicited cases. |
|
Total |
The total is computed by summing-up the sub-totals of Solicited and Non-solicited columns. |
|
Total events for <SOC> |
The count of Events is printed against respective column at the end of SOC grouping. |
|
Total events for <Drug> |
The count of Events is printed against respective column at the end of Drug grouping. |
|
Total of distinct cases for <Drug> |
The count of Distinct Cases is printed against respective column at the end of Drug grouping. |
Figure 7-2 Format for HCP Summary Tabulations
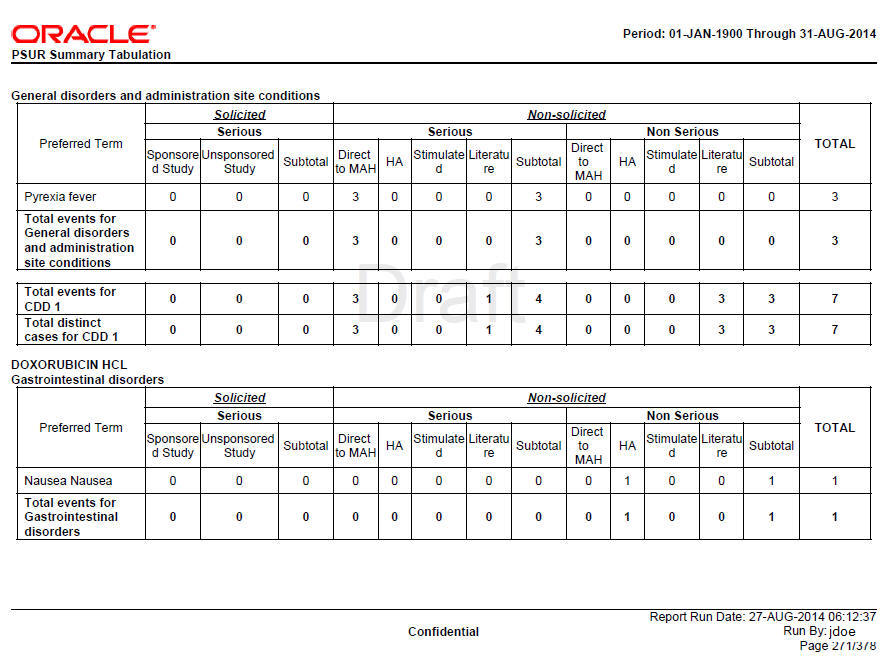
Description of "Figure 7-2 Format for HCP Summary Tabulations"
Parent topic: Summary Tabulations