7 Medical Device Defect Glossary
The JFMDA coding is enabled in the following case form sections:
- Product > Device > Device Component Information
- Product > Device > Health Impact Information
- Product > Device > Medical Device Problem Coding
- Product > Device > Evaluation / Investigation Code Information section
- Evaluation Coding – Method / Type
- Evaluation Coding – Result / Findings
- Evaluation Coding – Conclusion
- Event > Clinical Sign Coding
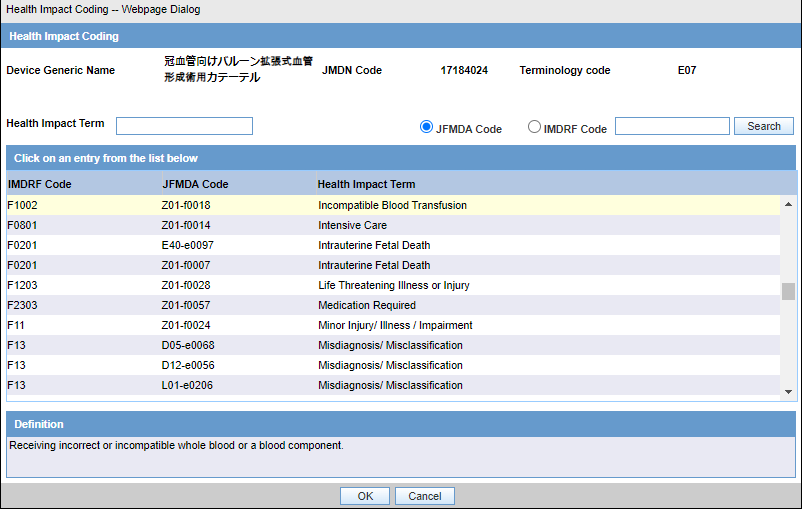
Japanese users can select the JFMDA code, and the corresponding IMDRF code is auto populated as shown in the following screenshot for Health Impact as an example. When the global users select the IMDRF code, the corresponding JFMDA code is not auto populated.
After the JFMDA code is added, if the global user accesses the same case and selects a different IMDRF code, the existing JFMDA code is overridden. It is recommended that the global user does not update the IMDRF codes when the JFMDA codes are already available.
After the IMDRF code is added, if the Japanese user accesses the same case in locally unlocked mode and selects a JFMDA code that does not match the IMDRF code, then a message is displayed as: This IMDRF code cannot be updated as the case is globally locked. Please select JFMDA code based on the existing IMDRF code to continue. (症例がグローバルロックされているため、この IMDRF コードを更新することができません。入力されたIMDRF コードに紐づいた JFMDA コードを選択してください”)
Figure 7-1 Health Impact
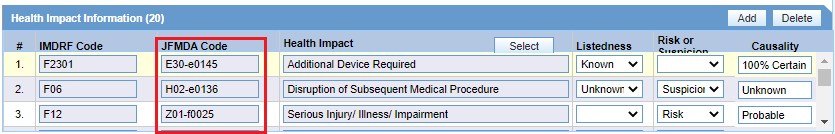
Figure 7-2 Health Impact lookup