Report
This section lists the fields descriptions and configuration steps for the Report tab.
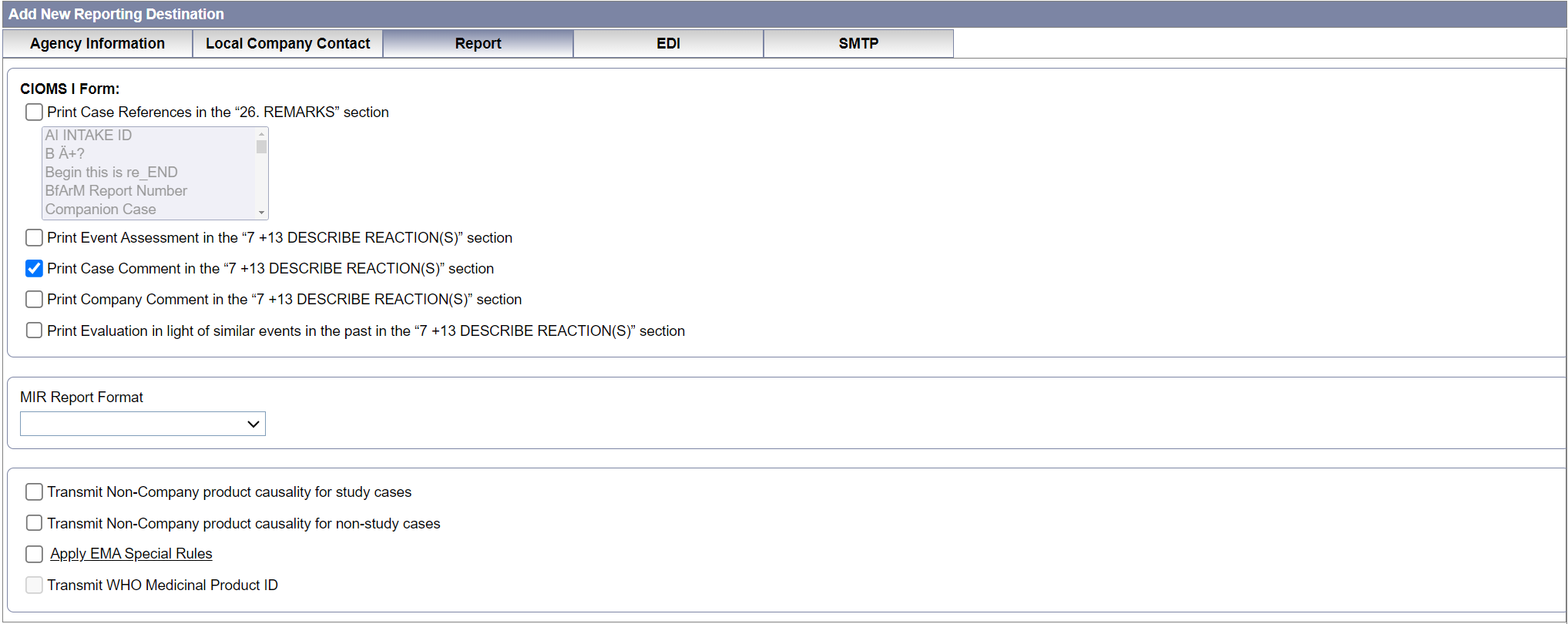
Field Descriptions
The following tables lists and describes the fields on the Report tab.
| Field or Control Name | Description |
|---|---|
| Print Case References in the “26. REMARKS” section | Enables you to select the case
references to be displayed in the CIOMS I report.
The selectable
values are populated with the English attributes as configured
in the |
| Print Event Assessment in the “7 +13 DESCRIBE REACTION(S)” section | Enables you to print the event assessment information in a tabular format in the CIOMS I report. |
| Print Case Comment in the “7 +13 DESCRIBE REACTION(S)” section | Enables you to print the case related
comments in the CIOMS I report.
The comment appeared
as entered in |
| Print Company Comment in the “7 +13 DESCRIBE REACTION(S)” section | Enables you to print the company
comments in the CIOMS I report.
The comment appeared
as entered in |
| Print Evaluation in light of similar events in the past in the “7 +13 DESCRIBE REACTION(S)” section | Enables you to print the analysis of the
similar events in the CIOMS I report prefixed with text
Analysis of Similar Events:.
The
comment appears as entered in |
| MIR Report Format | Enables you to select the MIR Report format from a drop-down list (PDF and XML). |
| Transmit Non-Company product Causality for study cases | When this checkbox is checked, the
causality for non-study, non-company products in the study cases is
transmitted in the E2B(R2) and E2B(R3) reports.
When this checkbox is unchecked (default), the causality for non-study, non-company products in the study cases is not transmitted in the E2B(R2, R3) reports. |
| Transmit Non-Company product causality for non-study cases | When this checkbox is checked, the
causality for suspect non-company products, non-study cases are
transmitted.
When this checkbox is unchecked (default), the causality for non-study, non-company products in the non-study cases is not transmitted in the E2B(R2, R3) reports. |
| Apply EMA Special Rules |
When this checkbox is checked, special rules are applied to modify certain data for EU cases if the reports are sent for Agency/Partners from non-EU countries or if the country is not part of the countries list approved by EMA. This checkbox impacts E2B(R2), R2B(R3) and CIOMS-I reports. When you click on the Apply EMA Special Rules hyperlink, a pop-up appears having details of the impacted profile and elements with rule description. |
|
Transmit WHO Medicinal Product ID |
Enables you to select whether to transmit WHO medicinal product ID or not in the E2B(R3) report. This checkbox is enabled when the Message Profile is set to any of the HL7 E2B(R3) profiles. Otherwise, the checkbox is unchecked and disabled. Note: This checkbox is added for future purposes. Currently there is no functionality associated with this checkbox. |
Parent topic: Configuring Reporting Destination