Adding Product Licenses
This screen helps capture License information (License specifics, associated with the License, Countries where the product is marketed or is under investigation). This data is reflected in multiple expedited and periodic reports and in case form-product information section.
Use the following procedure to add a product license.
- In the Business Configuration section, select Product and Licenses.
- In the left panel, select a filtering criterion.
- Expand the folders till you reach the license associated with a product.
- Select a license and click to view the license in the right panel.
- The system opens the following screen:
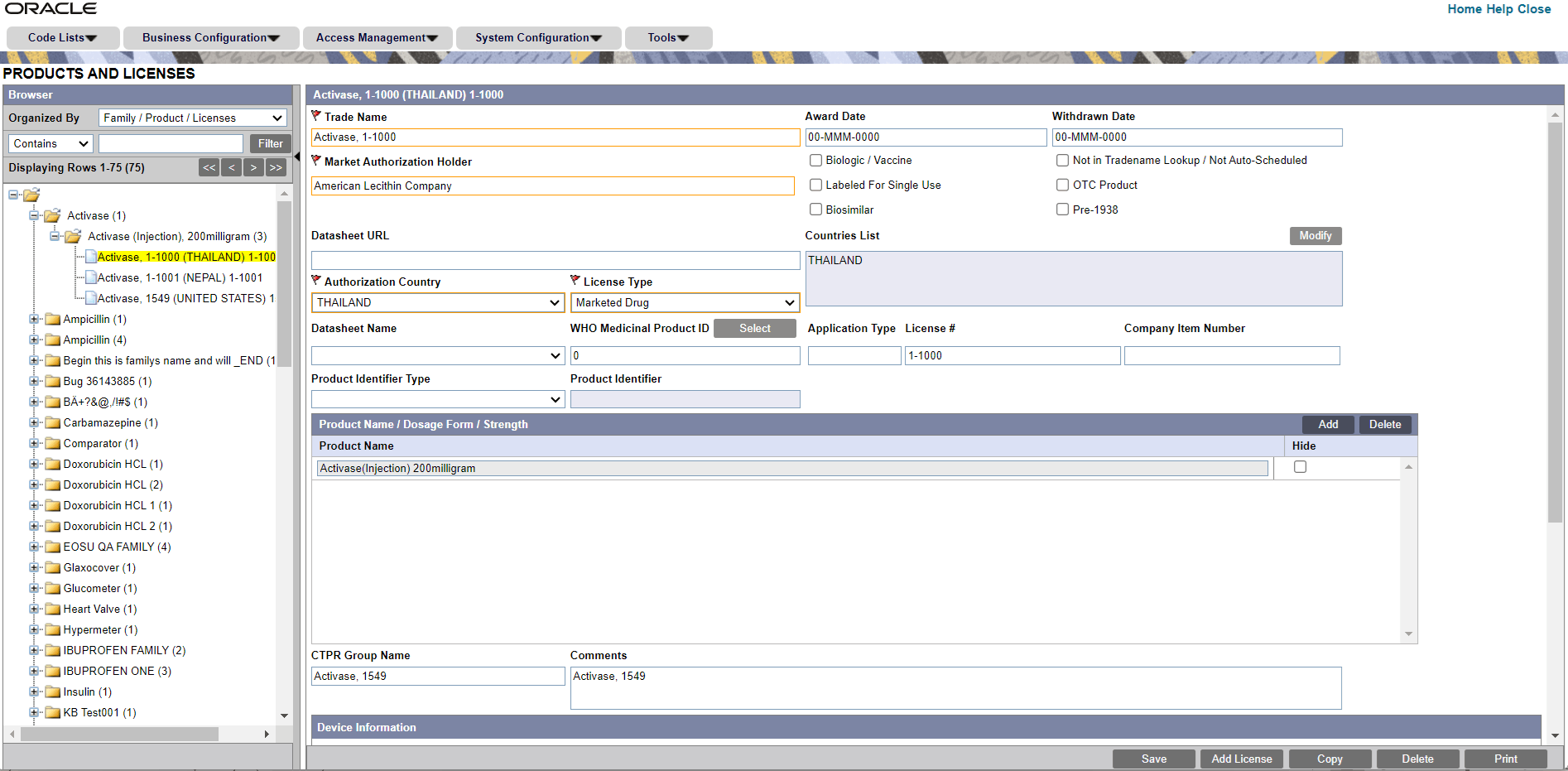
- Enter the Trade Name of the license.
- In the Manufacturer list, select the manufacturer of the product.
- Select the Authorization Country in which the license was issued.
- Select the License Type.. This is the type of license.
- To select the WHO Medicinal Product ID, click Select adjacent to this field. From the Drug Coding pop-up, select a drug to populate the WHO Medicinal Product ID.
- Enter the license number in License#.
- If this license is to be reported is under the PLA# and not the NDA#, select the Biologic/Vaccine checkbox. If this checkbox is selected, the PLA# (and not the NDA#) will be printed in section G5 of the MedWatch form.
- Specify if the drug is Labeled for Single Use or not.
- Specify if the drug has been bought as an Over-the-Counter (OTC) Product.
Note:
If the OTC Product checkbox is checked inBusiness Configuration > Products and Licensesfor a product whose Authorization Country is US and Withdrawn Date is blank, then the OTC Product checkbox in theCase Form > Analysis > Medwatchtab will be checked automatically on selecting the product license if the Initial Receipt Date is equal to or greater than the Award Date of the license. - Specify if the drug is Biosimilar or not.
- Specify if the drug is Pre-1938 or not.
- Under Award Date, enter the date the license was granted to the manufacturer.
- Enter the Withdrawn date, if applicable.
- Enter the Company item number in Company item number.
- Enter a URL reference for the license under Data Sheet URL (A URL reference might be a link to product label or product information).
- A world wide web address or an appropriate network path (For example: http://anydomainname/anypath or \\FILESERVER\LOCATION) can be entered in this field.
- Select Not in Tradename lookup/Not Auto-Scheduled if this license is not to be involved in reporting.
- In the Countries List, select the countries that define whether the case will be classified as domestic or foreign for regulatory report scheduling algorithm. The list of countries field in the product license screen used to display the ISO A3 codes assigned to each country. But since the new countries do not have ISO A3 codes, this field now displays the full country names with the semicolon as delimiter.
Tip:
To modify this list, use the Modify option (placed next to the Countries List). - Select the Data Sheet Name associated with the license, from the drop-down list.
- If you have a company product selected on the Drug/Device/Vaccine tab in Argus Case Form > Products, then the Product Identifier Type and Product Identifier fields are auto-populated based on the selected license. If the selected license does not have a Product Identifier Type and Product Identifier defined in Argus Console, then do not set the Product Identifier Type and Product Identifier fields.
- Click Add in the Product Name/Dosage Form/Strength to add a product to the License
Tip:
You can alternatively click Add License to create a new license.
Use Copy to make an editable copy of an existing license.
The Product Browser dialog opens. - Enter the name (partial or full) of the product and select Full Search.
- Select the appropriate product in the search results, and click Select. Enter all the required products in this manner.
Parent topic: Configuring Licenses