Understanding composite and regular datasources
Oracle Site Select supports use of composite (complex and structured) and regular (flat) datasources.
Composite
- Investigator
- Institution
- Trial
- site
- trial-site
This means that the Oracle Site Select composite data model imports five unique files: investigator, institution, trial, site, and trial-site that Oracle Site Select combines internally to create a single site row.
- Unique institution records combine with investigator records to form sites. Instead of having limited fields to import institution staff records, the investigator file can handle any number of institution staff in a nested structure of: first name, last name, email, phone, and role.
- The investigator file can import any number of investigator email addresses and is not limited to primary and secondary email.
- When creating a master list of sites for evaluation, if the datasource contains composite records, users can additionally filter by: therapeutic area, indication, drug class, and study phase.
- Oracle Site Select calculates an Investigator score for studies involving composite datasources. The score is the average of all site scores for the site's investigator, and it is listed for each of the investigator’s sites.
Note:
Oracle administrators manage composite datasources. Contact your Oracle Project Manager to manage configuration and import of data into these datasources.Regular
Regular datasources accept data in a flat, CSV (comma separated value) file format, such as an Excel spreadsheet. In this flat data model, each CSV row is a site and that row includes investigator, institution, and site data.
You can configure regular datasources and import data into regular datasources using the Oracle Site Select application. Once you've imported data into a new datasource, contact your Oracle Project Manager who can associate the new datasource to your studies.
Preferred sites: This type of source allows CRO preferred sites to bypass study parameter filters and move into the Master List. This option requires upload and mapping of a CSV format file with CRO preferred sites. The source file must contain a column labeled "preferred_site" and must specify TRUE or FALSE for each row in the source.
Pre-selected sites: You can also upload study-specific, pre-selected site lists and have only those sites included in the datasource displayed on the Master List. To use this functionality, the datasource must include a is_pre_selected_site column, which must specify TRUE for all sites in the datasource.
Viewing datasource content
For both regular and composite datasources, you’ll see a “Records” menu item on the datasource details page above the records table. If applicable to the datasource, you can select a page or advance through the available pages using the pagination control at the lower left of the records table.
You can also search the record grid. Enter a value in the search field, and Select performs a “fuzzy” search (approximate string match) as follows:
Regular datasource searches data available in the records table for the following columns:
- unique id column
- first_name
- last_name
- institution
- country
- pi_emails
- city
- npi
- master_profile_id
Composite datasource searches the following columns by entity:
- Investigator (unique id column, first_name, last_name, pi_emails, and npi)
- Institution (unique id column, institution, country, and city)
- Site (unique id column, investigator_id, institution_id, and master_profile_id)
- Trial (unique id column, protocol_title, and protocol_numbers)
- Trial-site (unique id column, site_id, and trial_id)
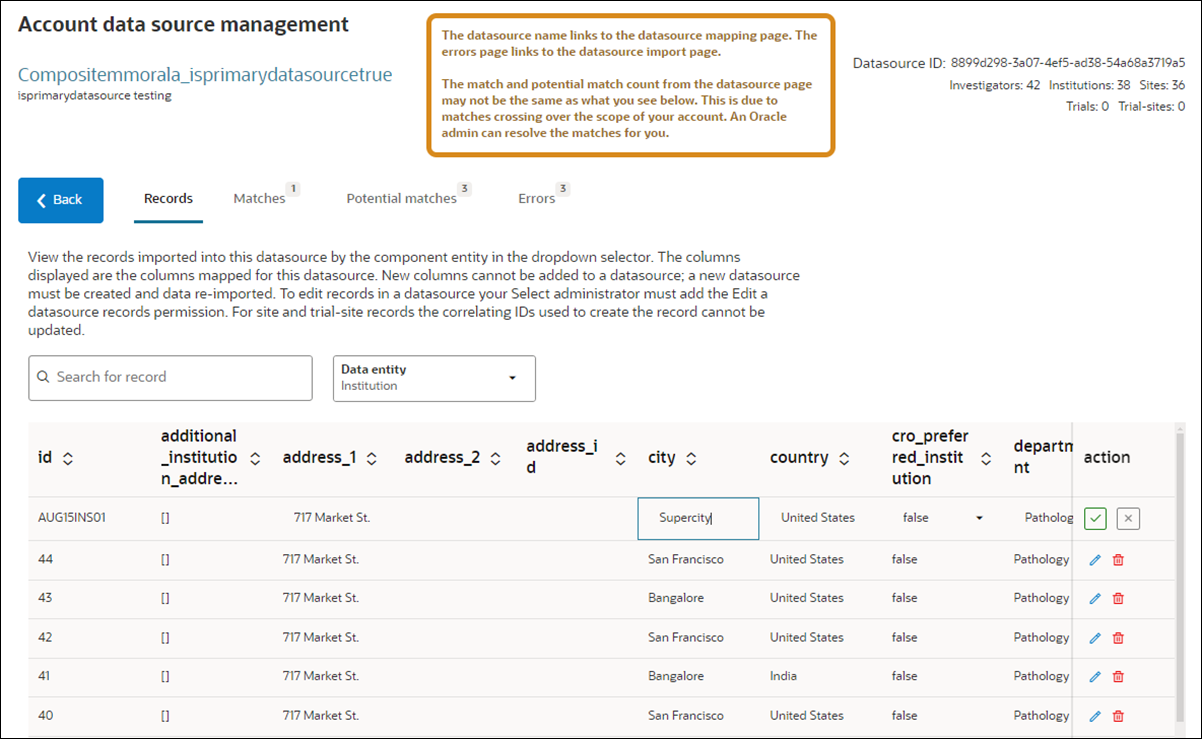
Viewing and modifying datasource records
Viewing records
When you view a regular or composite datasource, you’ll see all mapped data columns for that datasource with all rows of imported site records and the data imported for each site. For composite datasources only, use the Data entity filter (above the data table) to choose between the five composite datasource entity types (i.e., Investigator, Institution, Site, Trial, or Trial-site) and filter the data in the table to the selected type. For both regular and composite datasources, the unique ID column for each site row is the first column in the table. Where appropriate, you can hover over a cell to view a tooltip with the full content of the cell (e.g., long string array values).
Editing or deleting records
When you have Edit a datasource record permission, you’ll see a fixed Action column with Edit and Delete action icons at the far right of the records table. Note that this permission is a user role permission that doesn’t apply to Teams. The permission allows you to edit regular and composite datasource entity records of the following data types:
- String
- Boolean
- Datetime
- Integer and real (numeric)
- Text
To edit a record, click the Edit icon and click the column cell you want to update. You’ll see a border around that editable cell. When you’ve finished the update(s), return to the Action column and click Save or Discard to revert unsaved updates(s) and return to view mode.
You can also delete a record from a datasource, and you’ll need to confirm this action. For regular datasources, deleting a record removes the record from the datasource. For composite datasources, please plan carefully for the following outcomes:
- Deleting a composite investigator or institution record that has a site correlation will cascade delete the site and any affiliated trial-site record(s)
- Deleting a composite trial record that has a trial-site correlation will cascade delete the affiliated trial-site record(s)
Parent topic: Import and Manage Data