2 Business Configuration
This chapter lists the Japanese-specific (J-specific) features in the Business Configuration module of Argus Console.
2.1 Configuring Product Family
The following list indicates the changes in Product Family Configuration:
-
Comments (J) has been added below the English Comments area.
-
This field is displayed only to an Oracle Argus Safety Japan (Argus J) user when the Japanese module is enabled.
-
It is printed in the Product Family Configuration print PDF right after the English Comments field.
-
It is covered by the back-end PL/SQL APIs for Product Family Configuration data table updates and audit-logging.
2.2 Configuring Licenses
The following section describes the changes in License Configuration:
2.2.1 License Configuration - Argus J Specific Parameters
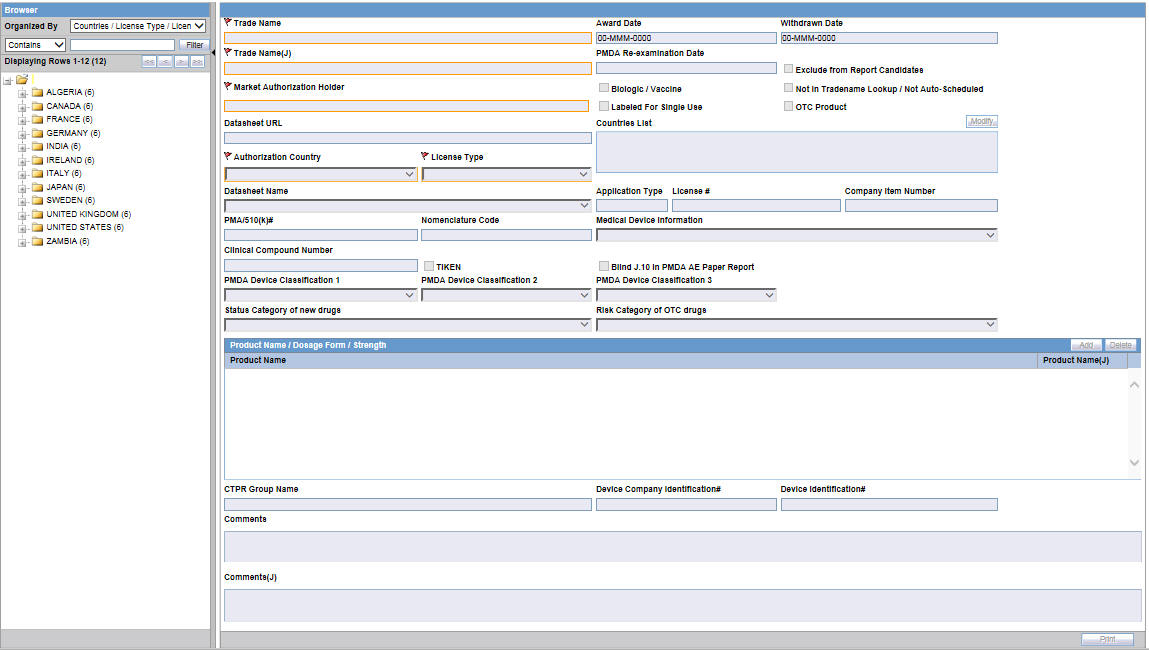
The following changes have been made for PMDA Device Reporting Support in Console:
-
The following drop-down lists have been added to Console > Business Configuration > Products and Licenses. These drop-down lists have the following options in the same order.
-
PMDA Device Classification 1:
High Level Controlled Medical Device (Class IV)
High Level Controlled Medical Device (Class III)
Controlled Medical Device
Generic Medical Device
Combination products (Drugs)
Combination products (Tissue-Engineered Medical Products)
Stand-alone software (Class IV)
Stand-alone software (Class III)
Stand-alone software (Class II)
PMDA Device Classification 2:
Biogenous
Specific Biogenous
Other
PMDA Device Classification 3:
Single Use Medical Device
Reiteration Use Medical Device
-
These drop-down lists have <Blank> as the default value.
-
These fields are displayed to only an Argus J user when Japanese module is enabled.
-
These fields are editable only when Authorization Country is selected as Japan and License Type is selected as either Marketed Device or Investigational Device.
-
The list options are displayed in English even to the Argus J user as this is an English base screen. The Japanese value specified for these options is used to populate them in PMDA Device Expedited Form 8 and 10.
-
Medical Device Information and Clinical Compound Number have been adjusted in the user interface of the application.
-
These three fields are printed in License Print PDF in three different rows, right below Clinical Compound Number field in alternate-colored rows thereafter.
-
These fields are audit-logged.
-
These fields are covered by the back-end PL/SQL APIs for License Configuration data table updates and audit-logging.
-
A new checkbox TIKEN is available. Any changes to this checkbox value are audit logged.
-
Blind J.10/J2.11 in PMDA AE Paper Report: This checkbox is disabled by default and shall be enabled only when the License country is Japan.
-
Status Category of new drugs: This list captures the Status category of new drugs. The data in this list is populated based on the data in the License Category code list.
-
Risk Category of OTC drugs: This list captures the Risk Category of over-the-counter (OTC) drugs. The data in this list is populated based on the data in the Risk Category of OTC Drug code list.
These new fields available in License Configuration print for both Print and Print All options. They also support the License/Product with Licenses copy functionality. Any changes to these fields value is logged for audit.
-
-
A separate Japanese Comments field is supported for the following in Console ' Business Configuration.
-
Comments (J) field has been added right below the English Comments area.
This field is displayed only to Argus J users when Japanese module is enabled.
It is printed in the License Configuration print PDF right after the English Comments field.
It is audit-logged and is also covered by the back-end PL/SQL APIs for License Configuration data table updates and audit-logging.
-
2.2.2 Literature Intake Updates
Following is the list of Literature Intake Updates:
-
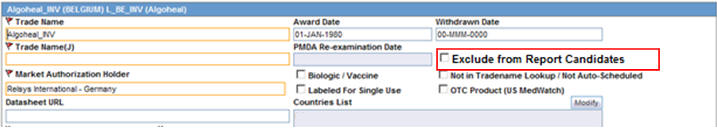
A new option Exclude from Report Candidates has been added to Console > Business Configuration > License Configuration as shown below.
-
This checkbox is displayed to only an Argus J user and when Japanese module is enabled.
-
By default, this field is unchecked.
-
This checkbox is enabled only when the Authorization Country is Japan.
-
This field value is printed in License Configuration print PDF.
-
Updates to this field value are audit-logged.
-
-
When the product is populated in the case created through J Literature Intake module, it populates only one record in the Case Form Products tab for each Product in the matching Product Family. If there are multiple Japanese licenses for a product, then the correct license is picked up based on the following logic:
-
License Authorization Country = Japan
-
Withdrawn date is blank or >= current system date
-
Hide checkbox is not selected for that product license combination
-
Not in Tradename lookup/Not Autoscheduled checkbox is not selected
-
Exclude from Report Candidates checkbox is not selected
-
If multiple licenses exist matching this criteria, then the Earliest awarded date license is considered
-
If multiple licenses still exist matching this criteria, then the license with the lowest internal sequence number is considered.
-
-
Following is the change in logic that is used to populate Japanese licenses on Event Assessment and PMDA tabs. There is no change in logic for other country licenses. Manually Added Products through Bookin or Case Form or Case Intake or Affiliate Event Acceptance:
-
If the user selects a Japanese license during product selection in Bookin / Case Form / Affiliate Event, then only that license is considered for Event Assessment and PMDA tabs irrespective of the value of Exclude from Report Candidates checkbox.
-
If the user selects a non-Japanese license during product selection in Bookin / Case Form / Affiliate Event, then only the Japanese licenses for which Exclude from Report Candidates checkbox is not selected is considered for populating Japanese licenses in Event Assessment and PMDA tabs.
-
-
Products Added through Literature Intake:
-
Only the Japanese licenses for which Exclude from Report Candidates checkbox is not selected are considered for populating Japanese licenses in Event Assessment and PMDA tabs.
-
-
Products Added through E2B Import:
-
Only the Japanese licenses for which Exclude from Report Candidates checkbox is not selected are considered for populating Japanese licenses in Event Assessment and PMDA tabs.
-
While identifying the product license to be used to populate the Products tab, only those Japanese licenses are used for which Exclude from Report Candidates checkbox is not selected.
-
This is applicable to all the E2B factory profile logic - ICH, FDA, EMEA and PMDA.
-
-
PMDA Event Assessment section on PMDA General tab:
-
Only the Japanese licenses for which Exclude from Report Candidates checkbox is not selected are considered for populating Japanese licenses in Event Assessment and PMDA tabs.
-
-
Manual Report Scheduling dialog > License # drop-down displays only those Japanese licenses which are available on Event Assessment tab.
-
The following change has been made while populating product licenses data in Case Form > Analysis tab > PMDA > General as well as Comment sub-tabs.
-
Marketed or Investigational Japanese Device Licenses is not populated, as PMDA General and Comments tab is not relevant for Device Reporting to PMDA.
-
Existing customer case data where Marketed or Investigational Japanese Device Licenses are already populated in PMDA General and Comments tab, has also been removed.
-
Removal of Marketed or Investigational Japanese Device Licenses from PMDA tab for existing customer data is audit logged with the SYSTEM user.
-
2.3 Configuring Studies
The user can now select a particular license that is then used to fetch the CCN and other related data.
The following changes have been made in Argus Console > Business Configuration > Study Configuration.
The application has been enhanced such that when user adds a J Drug to the Argus Console > Business Configuration > Study Configuration (J pop-up), the corresponding English Drug name is populated in the English Product name.
Note:
The same English product name is populated in the Case form English Product name (as explained below) when the corresponding study drug is added in the case.-
The application lists the above added J drugs in the study for which the English product name is not blank (or not default text J DRUG in DB ) in the corresponding English Study configuration screen. These products are listed in the existing Products grid of the corresponding English Study configuration screen.
-
The functionality of associating the J Drug in the study configuration with WHO drug remains intact.When the user tries to associate the WHO drug with the J Drug, by clicking the WHO Drug association button such that English Drug Name is not blank (or default text J DRUG) then the WHO Drug Browser opens with pre-populated English Drug Name (Populated from the English sub file) in the Trade Name (text box) of WHO Browser for user to perform a quick search .Also, when the user associates the J Drug to the WHO, the English product name is updated with the Product name returned from the WHO dictionary.
When the user adds a JDrug from J Drug Browser for with English Drug Name is blank, the existing behavior of populating J DRUG in the English Product Name has been retained.
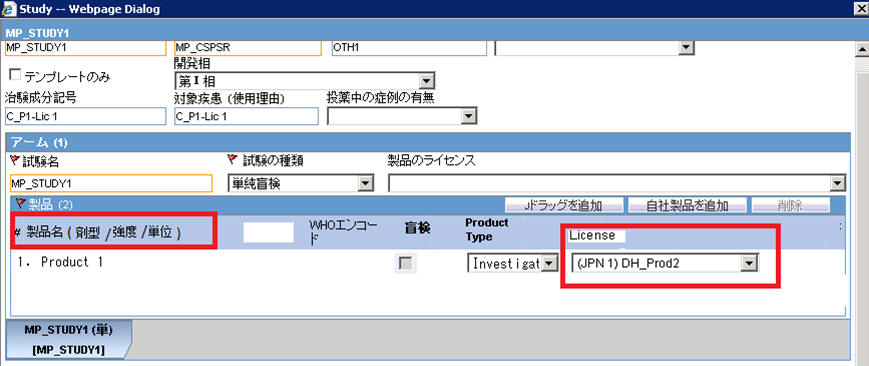
The Product Grid in both the English and J Study Configuration pop-up screens has been updated as follows:
-
Product, Dosage, Unit, and Formulation have been merged into one column called Product Name (Dosage Form / Strength / Unit), as shown below:
-
A new license selection drop-down list, License, has been added in the J pop-up Product Selection grid. This list contains all the J Licenses that are present for the respective product.
-
This license is used as the primary selected license when you select the product as the study drug in the Case Form, in the following format:
Trade name<space>(License Type)<space>Clinical compound Number or License Number
The License Type is printed as "MKT" for Marketed Drug/Device/Vaccine and "INV" for Investigational Drug/Device/Vaccine.
This license should be used as Primary license if the case is accepted as Initial/Follow-up from E2B, Literature Intake or Case Intake.
-
A new study configuration field, Notification number, has been added in the J pop-up Product Selection grid to capture the notification number. The notification number is the unique number allocated by PMDA to each study for a particular Clinical compound number.
Description of the illustration ''fig4.jpg''
Description of the illustration ''fig5.jpg''
You must enter numeric data in this field.
This new field is available in Study Configuration print for both Print and Print All options. It also supports the Study copy functionality. Any change to this field value is logged for audit.
2.4 Configuring Expedited Reporting Rules
Following is the list of changes in Expedited Reporting Rules Configuration:
-
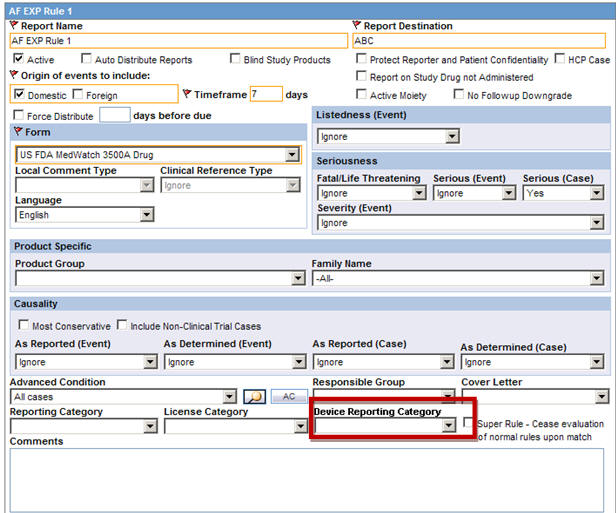
A new drop-down list Device Reporting Category has been added to Console > Business Configuration > Expedited Reporting Rules screen as follows:
-
This field is displayed to English as well as Japanese users only when Japanese module is enabled.
-
This drop-down displays the English values as specified in the Device Reporting Category Code List and are marked as Display.
-
It contains <Blank> as the first option and it is also the default value.
-
This field is printed in Expedited Report Rules Print PDF right below License Category field in alternating colored row.
-
This field is audit-logged.
-
It is covered by the back-end PL/SQL APIs for Expedited Reporting Rules data table updates and audit-logging.
-
-
A new option Urgent Report is available in the Reporting Rule Print. Selecting this checkbox will mark a report as urgent. The new option supports the copy functionality and changes to it will be logged for audit.
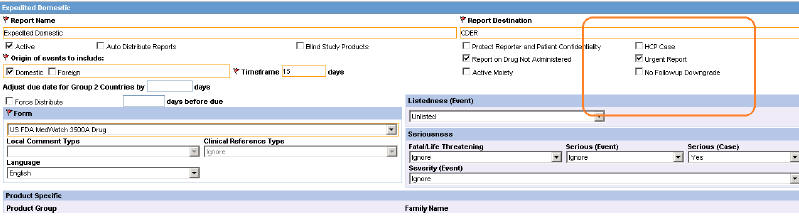
Description of the illustration ''fig6.jpg''