Oracle® Argus Safety
Quickstart Guide
Release 8.1.1
E90745-05
March 2021
Logging in
-
Open Microsoft Internet Explorer and enter the URL for Argus Safety.
-
On the Argus login screen, enter your username and password.
-
Select the appropriate database from the Database drop-down list and click Login.
The Home/Personal Argus Status page is displayed.
For more information, see the section Logging In and Out in the Oracle Argus Safety User's Guide.
Entering Case Data
Creating a New Case
-
On the Home page, hover over the Case Actions menu and click New.

-
On the Initial Case Entry page, enter the mandatory information (fields with a red flag) and click Continue.
-
On the BookIn screen, check the applicable checkboxes and click BookIn.
-
If Argus has been configured to generate a case number automatically, it is generated now.
If the case number is not automatically generated, enter it according to your company guidelines.
-
When the case creation message appears, click Yes.
Note: If you click Yes, the Case Form page appears. You can now enter case data.
If you click No, the case is saved and closed. You can open the case and enter data at any time.
For more information, see Creating a New Case in the Oracle Argus Safety User's Guide.
Finding an Existing Case
-
On the Home page, hover over the Case Actions menu and click Open.

-
On the Case Search Criteria page, select the applicable search criteria and click Search.
-
When the system displays the search results, locate the case in the list.
-
Click the link associated with the Case ID to open that case in the Case Form.
For more information, see Finding and Opening Existing Cases of the Oracle Argus Safety User's Guide.
Updating a Case
-
Case data is organized on four tabs: General, Patient, Products, and Events.
-
These tabs have sub-tabs and sections that further organize the information collected. Use the
 button on the right side of the screen to minimize or maximize each section.
button on the right side of the screen to minimize or maximize each section. -
Tabbing from field to field activates any auto-calculated fields.
-
When you finish entering information for a section, tab, or sub-tab, click the Save icon at the top of the page.
See the following sections for information on using the tabs.
Entering General Data

For basic information on using the General tab, see General Tab in the Oracle Argus Safety User's Guide.
For details on entering data in each section of the General tab, see:
Entering Patient Data
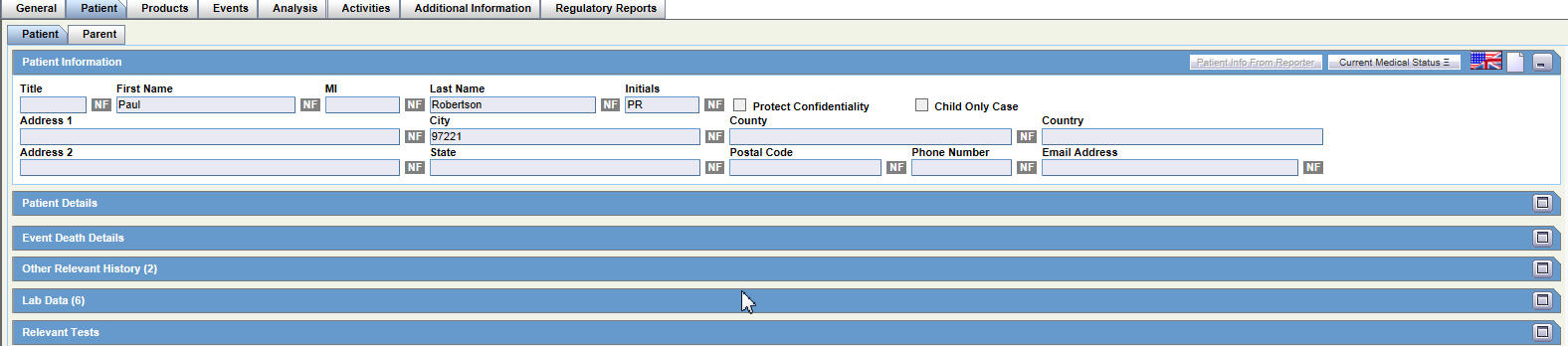
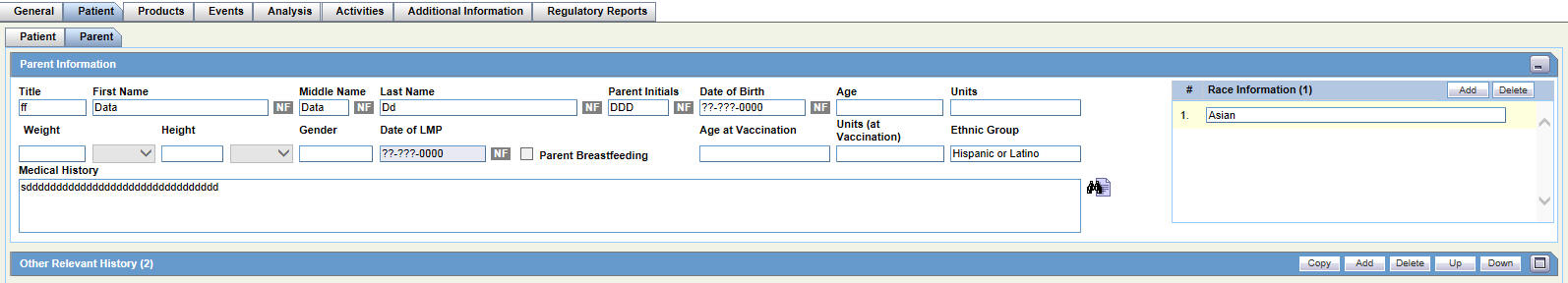
For general information on entering Patient data, see Patient Tab in the Oracle Argus Safety User's Guide.
The Patient tab has two sub-tabs.
-
Patient Tab
-
Parent Tab
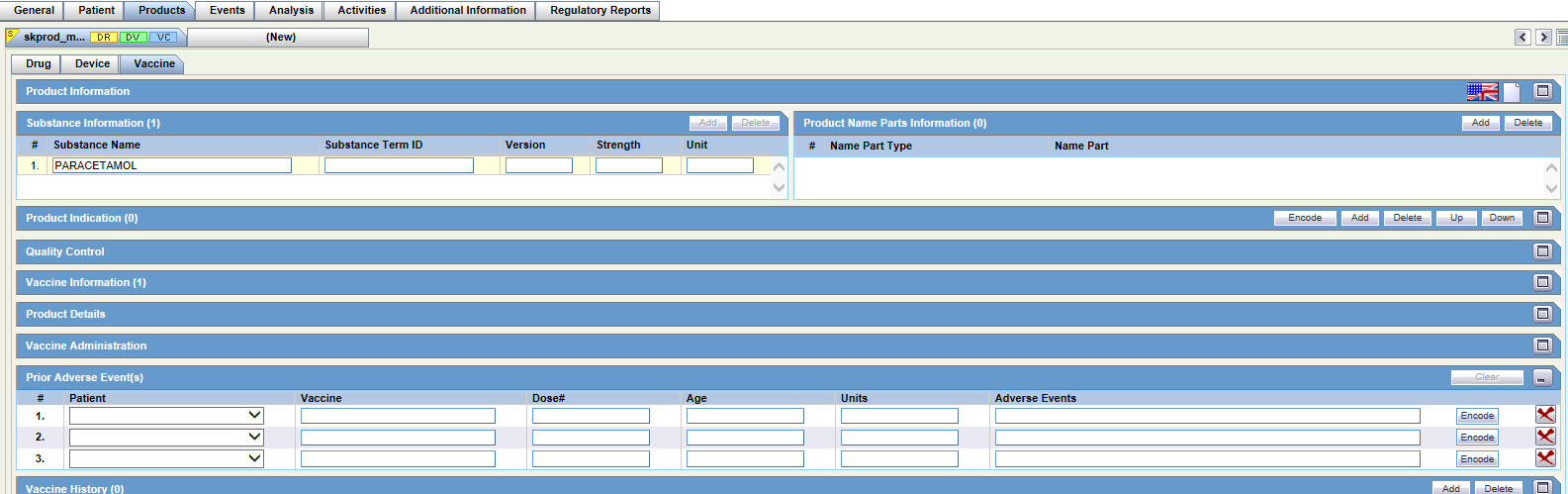
Entering Products Data
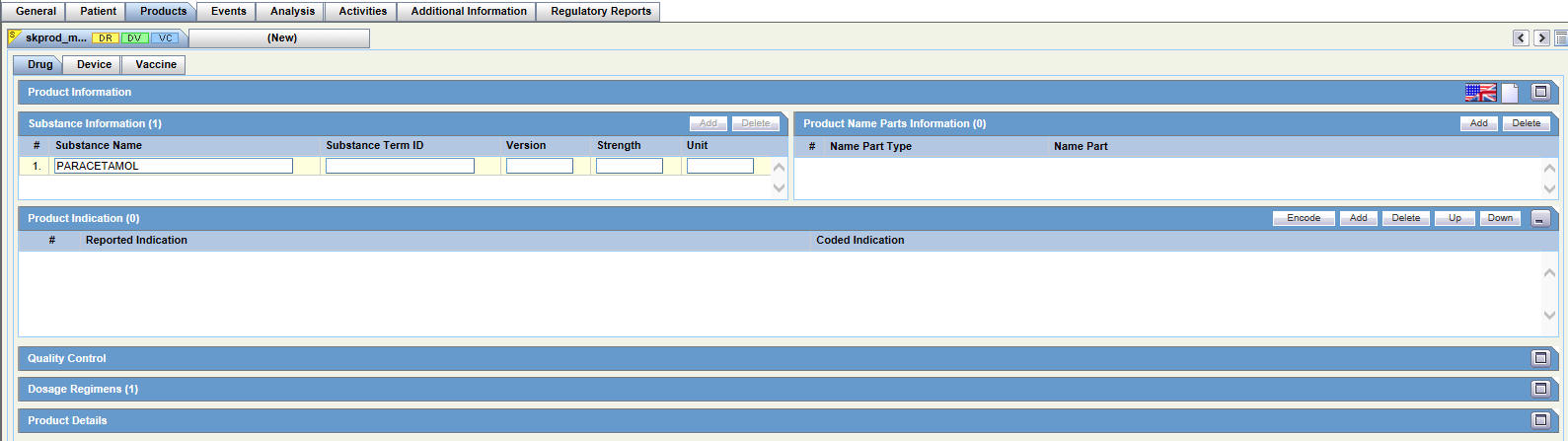
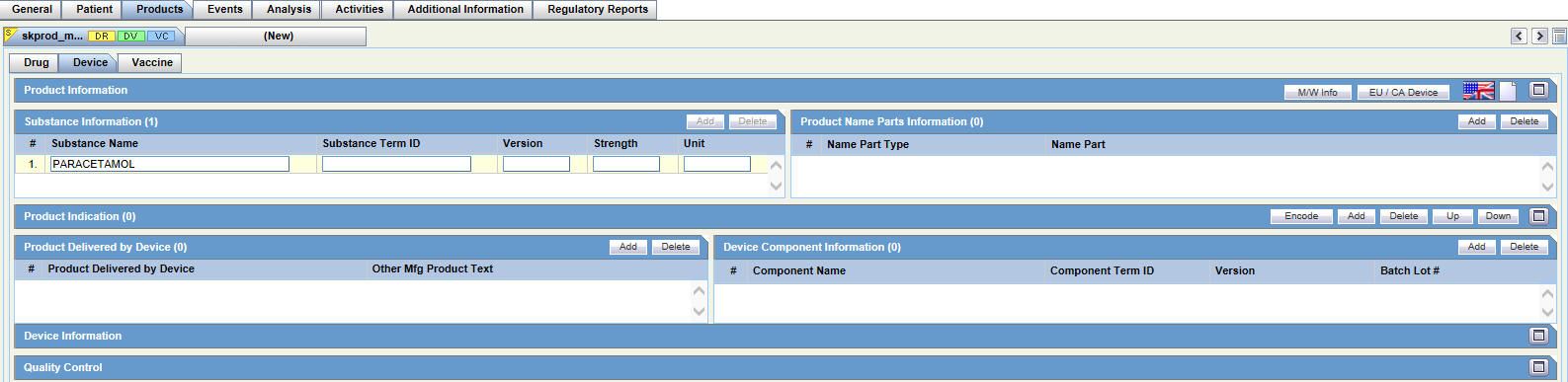
For general information on entering Products data, see Products Tab in the Oracle Argus Safety User's Guide.
The Products tab has the following sub-tabs:
-
Drug Tab
-
Device Tab
-
Vaccine Tab
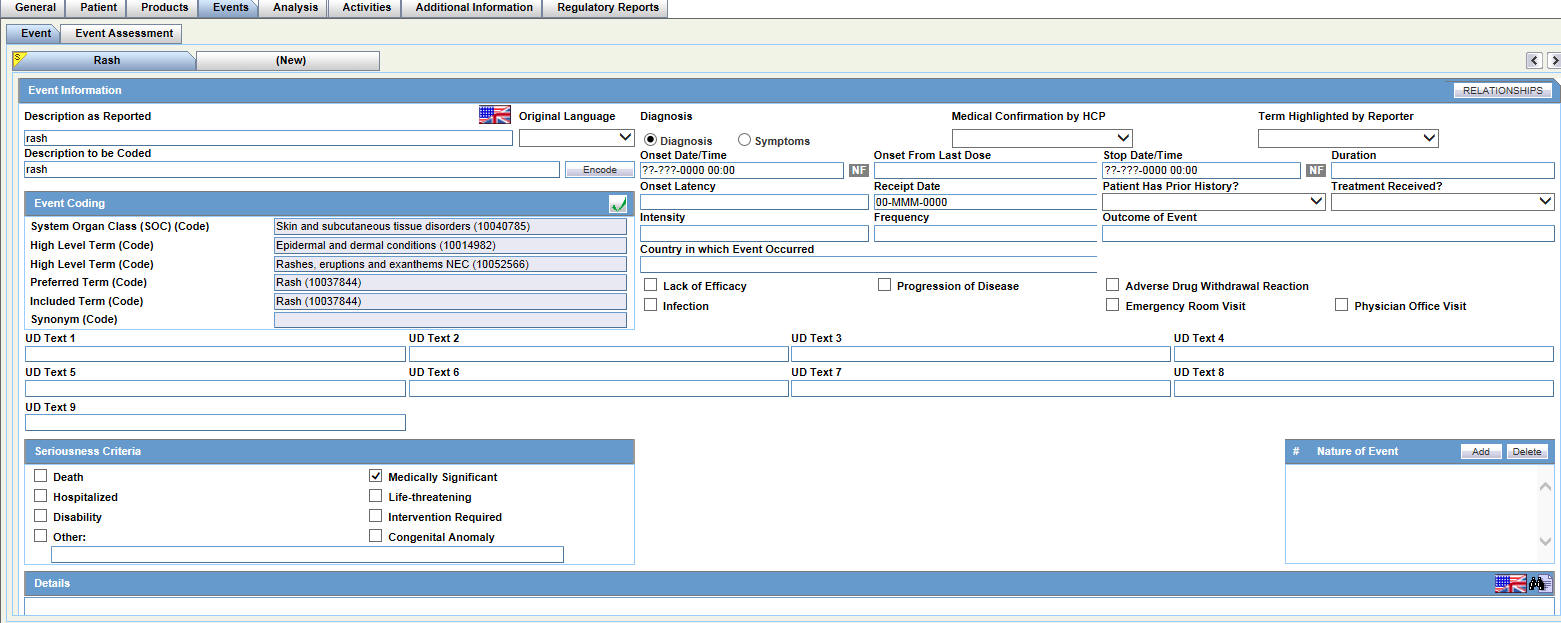
Entering Events Data
For general information on entering Events data, see Events Tab in the Oracle Argus Safety User's Guide.
The Events tab has the following sub-tabs:
-
Event Tab
-
Event Assessment Tab
Viewing Updates made to your Case
-
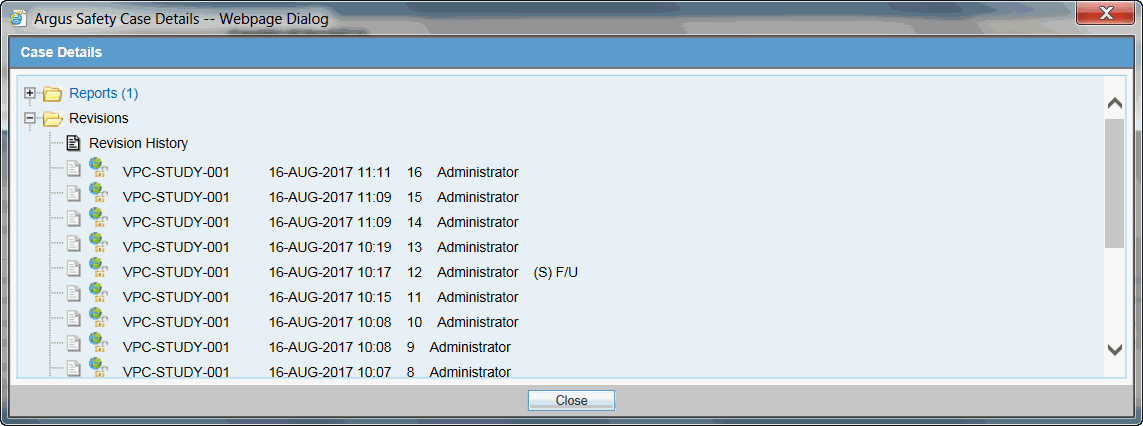
Open an existing case, hover over the Case Actions menu and click Case Revisions.
-
On the Argus Safety Case Details dialog box screen, click Revisions.
-
From the Revision History, click on a specific revision to view the changes made.
Attaching Additional Information to your Case
-
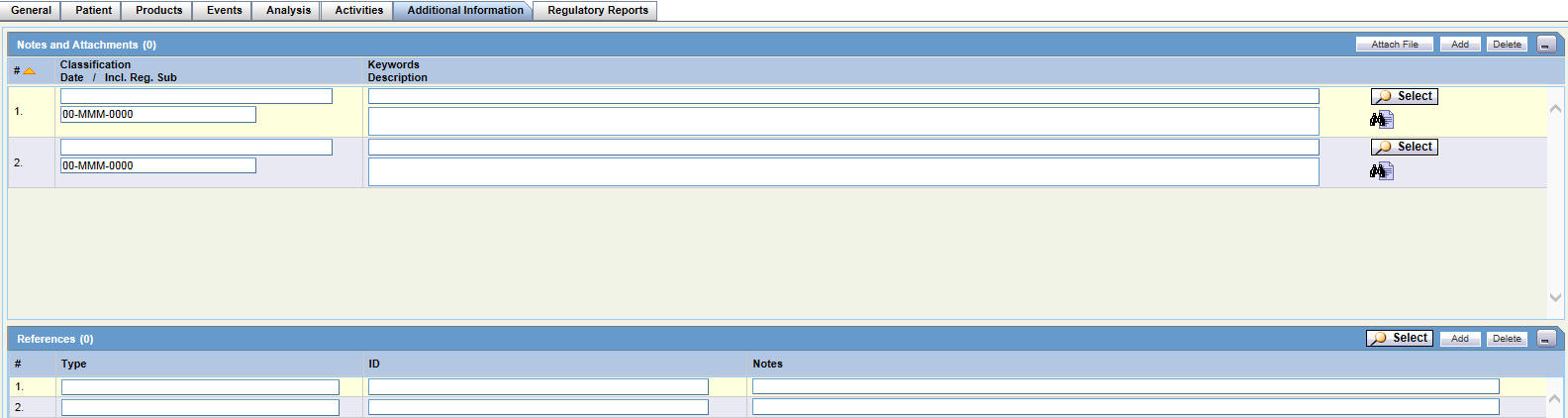
On the Case Form page, click the Additional Information tab.
-
Enter information in the Notes and Attachments and References sections.
For basic information on using the Additional Information tab, see Additional Information Tab in the Oracle Argus Safety User's Guide.
Reviewing your Cases
Viewing New Cases in the Worklist
-
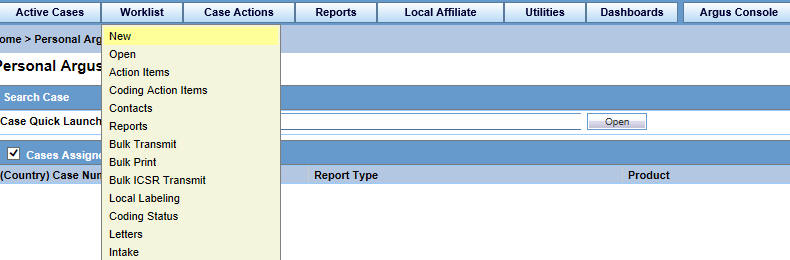
On the Home page, hover over the Worklist menu and click New.
-
On the New page, enter the required information and click Search.
For details on entering data in each section of the New page, see:
Note: Once you have accepted the case, it is Open.
Finding a Case Assigned to you
-
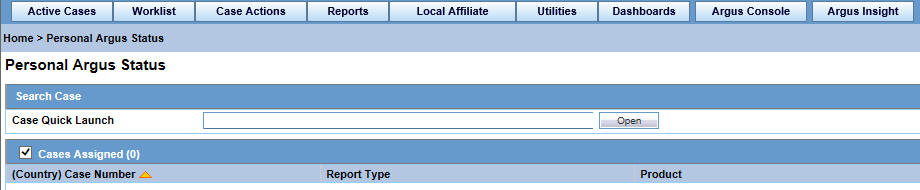
On the Home/Personal Argus Status page, check the Cases Assigned checkbox.
The list of cases assigned to you is displayed.
For more information, see Case Workload in the Oracle Argus Safety User's Guide.
If the Personal Argus Status Page is not your default home page, go to Utilities and click Personal Argus Status.
Viewing your Pending Action Items
-
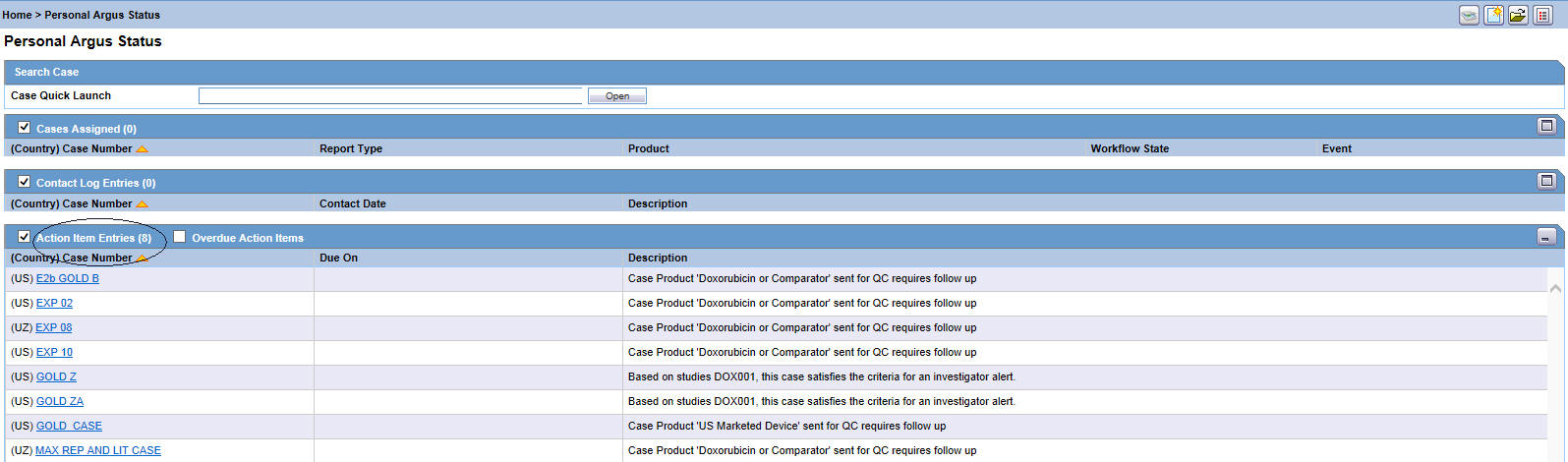
On the Home/Personal Argus Status page, check the Action Item Entries checkbox.
The list of your pending action items is displayed.
For more information, see Personal Argus Status in the Oracle Argus Safety User's Guide.
Viewing your Worklist Action Items
-
On the Home page, hover over the Worklist menu and click Action Items.
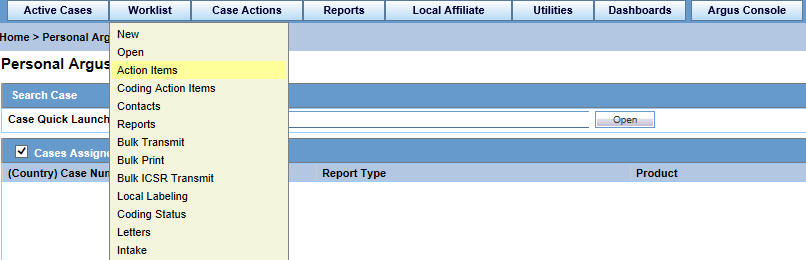
-
On the Action Items page, enter the required information and click Search.
-
Click Print List.
For more information, see Action Items in the Oracle Argus Safety User's Guide.
Viewing your Coding Action Items
-
On the Home page, hover over the Worklist menu and click Coding Action Items.
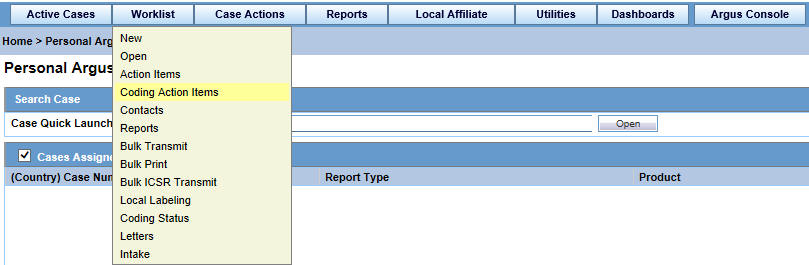
-
On the Coding Action Items page, enter the required information and click Search.
-
Click Print List.
For more information, see Coding Action Items in the Oracle Argus Safety User's Guide.
Performing a Medical Review
-
When you finish entering information for a section, tab, or sub-tab, click the Save icon at the top of the page.
Reviewing Case Narrative

For basic information on using the Analysis tab, see Analysis Tab in the Oracle Argus Safety User's Guide.
For details on reviewing and entering data in each section of the Analysis tab, see:
Reviewing MedWatch Information
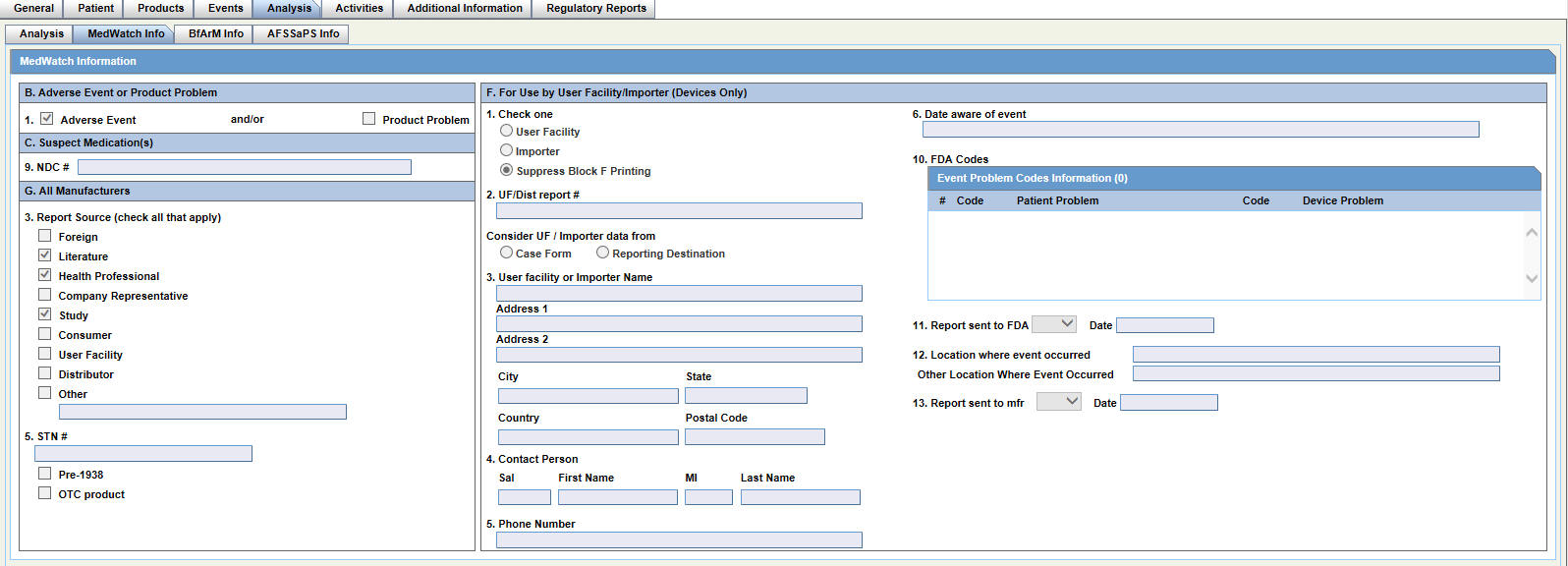
For details on reviewing and entering data in each section of the MedWatch Info tab, see:
Reviewing BfArM Information
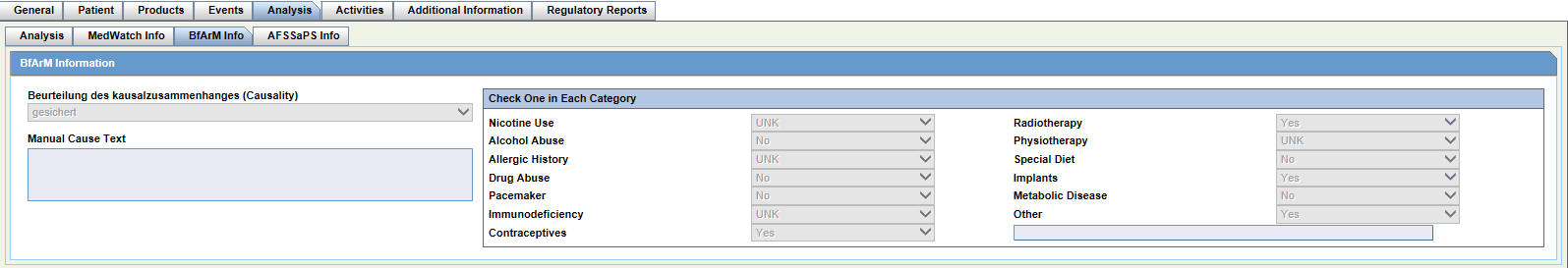
For details on reviewing and entering data in each section of the BfArM tab, see:
Managing your Report Submissions
The following procedures describe tasks for Expedited Reports. You can also schedule, review, generate, and transmit Periodic reports in a similar manner.
Scheduling an Expedited Report
-
Open the case for which you want to schedule a report.
-
On the Case Form page, click the Regulatory Reports tab.
-
On the Regulatory Reports page, click Schedule New Report.
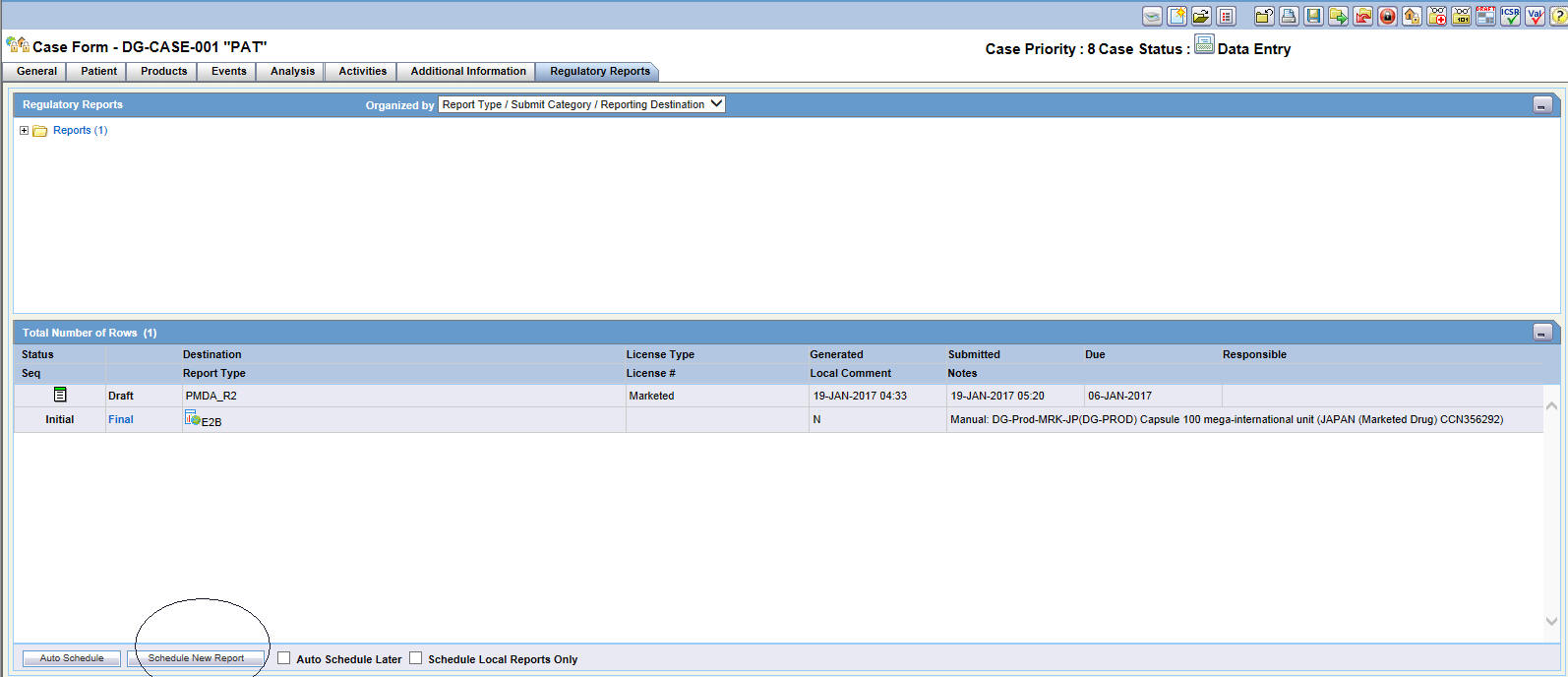
-
On the Schedule New Expedited Report page, enter the required information and click OK.
-
Click the Save icon at the top of the page.
For details on using Scheduling Reports, see Scheduling Reports in the Argus Safety User's Guide.
Note: This section also contains instructions for Auto-scheduling Reports.
Reviewing a Draft Report
-
Open the case for which you want to schedule a report.
Note: You can only view a Draft report if the relevant case is unlocked.
-
On the Case Form page, click the Regulatory Reports tab.
-
From the Total Number of Rows tab, locate the relevant report and click Draft to view the report.
For details on viewing and generating Reports, see Generating Reports in the Argus Safety User's Guide.
Generating an Expedited Report
-
Open the case for which you want to schedule a report.
Note: Verify that the relevant case has been locked and the required report has been scheduled.
-
On the Case Form page, click the Regulatory Reports tab.
-
From the Total Number of Rows tab, locate the relevant report and click Final to generate the report.
For details on using Generating Reports, see Generating Reports in the Oracle Argus Safety User's Guide.
Transmitting a Report
-
On the Case Form page, click the Reports tab, then select Compliance, and then Periodic.
-
On the Periodic Reports page, locate the relevant report and click on the Status of the report.
-
On the Report Details page, click Transmission and then click Transmit.
-
On the Transmit to Recipients page, enter the required information and click Transmit.
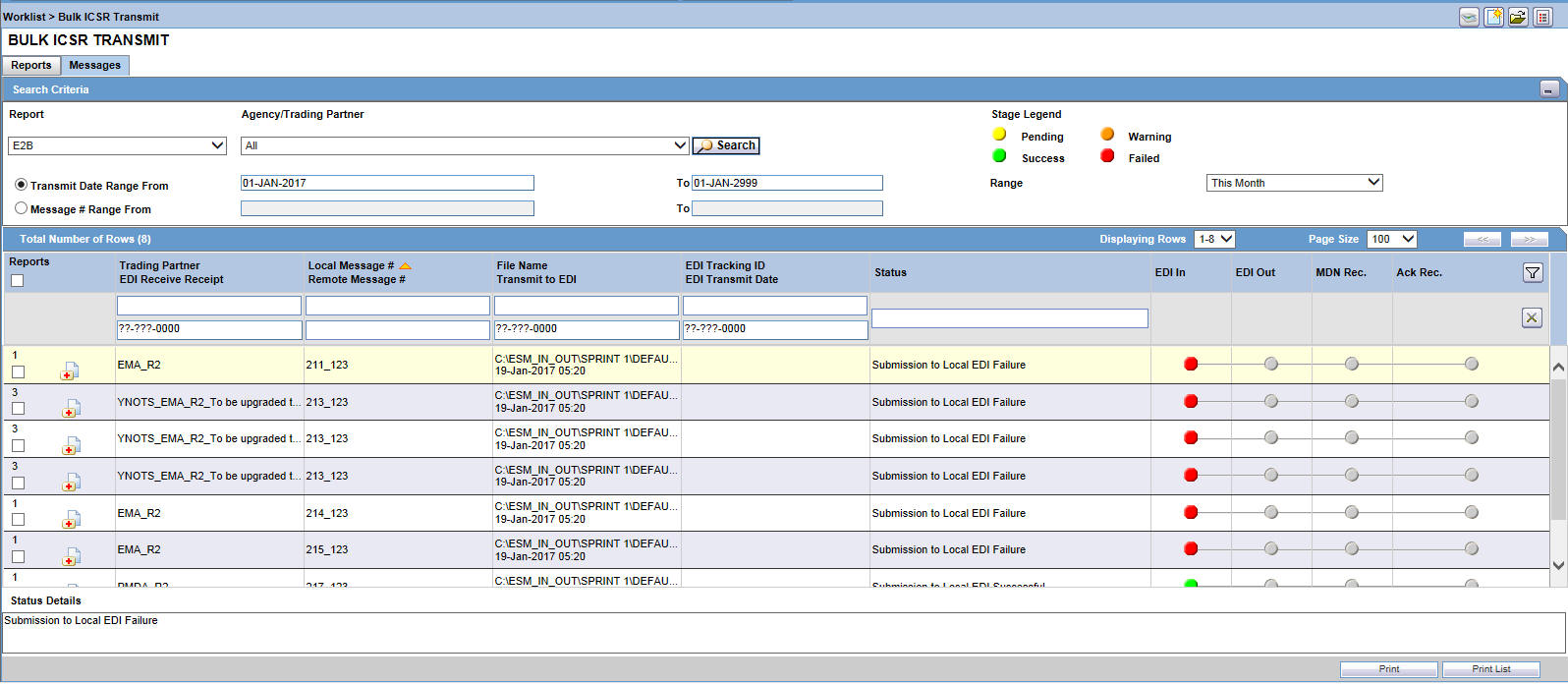
You can also transmit several reports together using the Bulk Transmit option.
-
On the Home page, hover over the Worklist menu and click Bulk Transmit.
-
On the Bulk Transmit page, enter the required information and click Search.
For details on entering data in each section of the Search Case page, see:
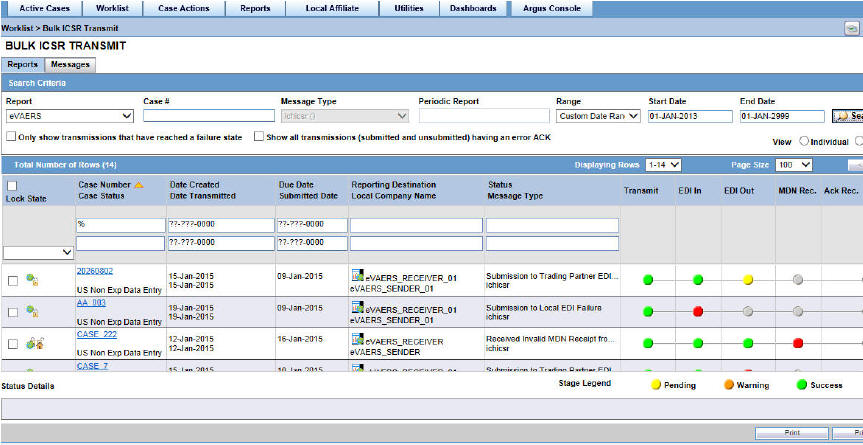
Tracking your ICSR Report Submissions
-
On the Home page, hover over the Worklist menu and click Bulk ICSR Transmit.
For general information on tracking your submissions, see Bulk ICSR Transmit in the Oracle Argus Safety User's Guide.
-
The Bulk ICSR Transmit screen has the following sub-tabs:
-
Reports Tab
-
Messages Tab
-
Finding an Overdue Report
-
On the Home page, hover over the Worklist menu and click Reports.
-
Click on the field Days Past Due on the right of the Total Number of Rows and if needed click again to show Days Past Due in descending order (arrow pointing downwards).
-
To view all reports select ALL at top right of the screen.
-
Click the Search button, at the top left of the screen. Overdue cases are all those reports shown as greater than 0 Days Past Due and greater than today's date.
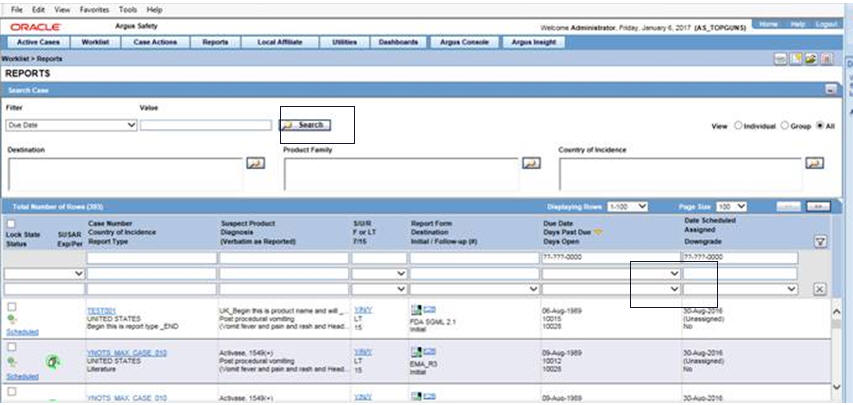
For details on entering data in each section of the Total Number of Rows tab, see Total Number of Rows.
Documentation Accessibility
For information about Oracle's commitment to accessibility, visit the Oracle Accessibility Program website at http://www.oracle.com/pls/topic/lookup?ctx=acc&id=docacc.
Oracle customers that have purchased support have access to electronic support through My Oracle Support. For information, visit http://www.oracle.com/pls/topic/lookup?ctx=acc&id=info or visit http://www.oracle.com/pls/topic/lookup?ctx=acc&id=trs if you are hearing impaired.
Oracle Argus Safety Quickstart Guide, Release 8.1.1
E90745-05
Copyright © 2016, 2021, Oracle and/or its affiliates.
This software and related documentation are provided under a license agreement containing restrictions on use and disclosure and are protected by intellectual property laws. Except as expressly permitted in your license agreement or allowed by law, you may not use, copy, reproduce, translate, broadcast, modify, license, transmit, distribute, exhibit, perform, publish, or display any part, in any form, or by any means. Reverse engineering, disassembly, or decompilation of this software, unless required by law for interoperability, is prohibited.
The information contained herein is subject to change without notice and is not warranted to be error-free. If you find any errors, please report them to us in writing.
If this is software or related documentation that is delivered to the U.S. Government or anyone licensing it on behalf of the U.S. Government, then the following notice is applicable:
U.S. GOVERNMENT END USERS: Oracle programs (including any operating system, integrated software, any programs embedded, installed or activated on delivered hardware, and modifications of such programs) and Oracle computer documentation or other Oracle data delivered to or accessed by U.S. Government end users are "commercial computer software" or "commercial computer software documentation" pursuant to the applicable Federal Acquisition Regulation and agency-specific supplemental regulations. As such, the use, reproduction, duplication, release, display, disclosure, modification, preparation of derivative works, and/or adaptation of i) Oracle programs (including any operating system, integrated software, any programs embedded, installed or activated on delivered hardware, and modifications of such programs), ii) Oracle computer documentation and/or iii) other Oracle data, is subject to the rights and limitations specified in the license contained in the applicable contract. The terms governing the U.S. Government's use of Oracle cloud services are defined by the applicable contract for such services. No other rights are granted to the U.S. Government.
This software or hardware is developed for general use in a variety of information management applications. It is not developed or intended for use in any inherently dangerous applications, including applications that may create a risk of personal injury. If you use this software or hardware in dangerous applications, then you shall be responsible to take all appropriate fail-safe, backup, redundancy, and other measures to ensure its safe use. Oracle Corporation and its affiliates disclaim any liability for any damages caused by use of this software or hardware in dangerous applications.
Oracle and Java are registered trademarks of Oracle and/or its affiliates. Other names may be trademarks of their respective owners.
Intel and Intel Inside are trademarks or registered trademarks of Intel Corporation. All SPARC trademarks are used under license and are trademarks or registered trademarks of SPARC International, Inc. AMD, Epyc, and the AMD logo are trademarks or registered trademarks of Advanced Micro Devices. UNIX is a registered trademark of The Open Group.
This software or hardware and documentation may provide access to or information about content, products, and services from third parties. Oracle Corporation and its affiliates are not responsible for and expressly disclaim all warranties of any kind with respect to third-party content, products, and services unless otherwise set forth in an applicable agreement between you and Oracle. Oracle Corporation and its affiliates will not be responsible for any loss, costs, or damages incurred due to your access to or use of third-party content, products, or services, except as set forth in an applicable agreement between you and Oracle.