6 Periodic Benefit Risk Assessment Report
This chapter contains the following topics:
6.1 Report Objective
The Periodic Benefit Risk Assessment Report (PBRER) is a standard for periodic benefit-risk evaluation reporting on marketed products (including approved drugs that are under further study) among the ICH regions.
When a medicinal product is approved for marketing, demonstration of safety and efficacy are generally based on data from a limited number of patients, with many studied under the controlled conditions of randomized trials. Higher risk subgroups and patients with concomitant illnesses that require use of other drugs are often excluded from clinical trials, and long-term treatment data is limited. Moreover, patients in trials are closely monitored for evidence of adverse events.
In clinical practice, monitoring is less intensive, a broader range of patients are treated (age, co-morbidities, drugs, genetic abnormalities, and so on), and events too rare to occur in clinical trials (such as severe liver injury) may be observed. These factors underlie the need for continuing analysis of relevant safety, efficacy, and effectiveness information throughout the lifecycle of a medicinal product promptly and periodically, for an overall assessment of the accumulating data.
The PSUR provides a comprehensive picture of the safety of approved medicinal products, as the assessment of the risk of a medicinal product is most meaningful when considered in light of its benefits. The PBRER provides greater emphasis on benefits, particularly when risk estimates change, and places greater emphasis on the cumulative knowledge regarding a medicinal product, while retaining a focus on new information.
Oracle Argus Safety customers can use the Periodic Safety Update Report configuration screen for defining the query criteria required for PBRER Section 6.2 - Cumulative Summary Tabulations of Serious Adverse Events from Clinical Trials and Section 6. 3 - Cumulative and Interval Summary Tabulations from Post-Marketing Data Sources.
The Periodic Benefit Risk Assessment Report contains the following tabulation sections:
-
Cumulative Tabulations of Serious Adverse Events from Clinical Trials
-
Number of ADR from Post Marketing Sources
6.2 Configuring Argus Safety for PBRER
To specify the inclusion of PBRER sections:
-
Select the Summary Tabulation tab from the Argus Safety ICH PSUR Configuration screen.
-
To include Section 6.2, select the Include Section 6.2 checkbox. The system does not use other query criteria available under Section 6.2.
-
To include Section 6.3, select the Include Section 6.3 checkbox. From the other query criteria available, only Case Classification and Observe Study Type are used for identifying the Non-Interventional studies.
-
The system populates Case Classification with values from the Case Classification code list.
-
The system populates Observe Study Type with values from the Case Classification code list where E2B code values are not null.
-
The Cumulative Start Date in Section 6.2 and Section 6.3 is separate from the Include Section 6.2 and Include Section 6.3 checkboxes.
-
The system ignores all other configuration parameters on this tab except PBRER configuration, Include Summary of Cases Missing Assessments, and Include Summary of Unlocked Cases.
Figure 6-1 ICH PSUR Summary Tabulations Tab
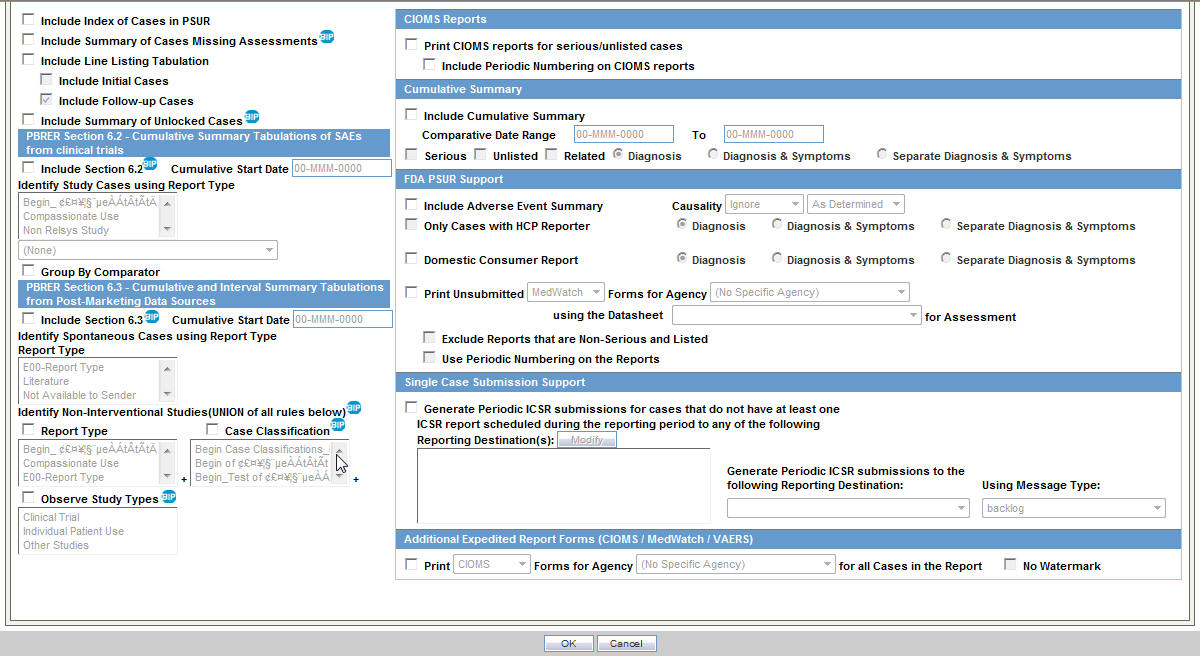
Description of ''Figure 6-1 ICH PSUR Summary Tabulations Tab''
6.2.1 Product Types in Study Configuration for Supporting PBRER and DSUR
To support PBRER and DSURs, the Product Type attribute of the Argus Console Study Configuration page displays the following values along with Investigational Product, Comparator, and Placebo:
-
No Study Drug Given: This value lets you classify an event into the No Study Drug Given category if a serious adverse event occurs even before a patient has started taking any study drug.
-
Additional Study Drug: These are additional or background drugs that are given as part of a combinational therapy. These drugs are generally given with both investigational products, and comparators. Additional study drugs do not impact the case categorization but are attributes for this configuration in the product temp table.
6.3 PBRER Section 6.2 - Cumulative Summary Tabulations of Serious Adverse Events from Clinical Trials
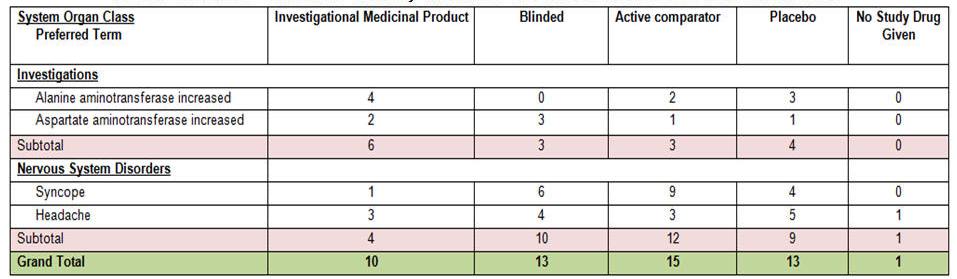
To support Section 6.2 in PBRER in tabulating based on medicinal product, active comparators, and placebo, the Argus Console Study Configuration is used to identify the a product type as Placebo, Comparator, or an Investigational Medicinal Product. The Argus Console Study Configuration classifies the counts of the various product types.
The system evaluates the list of cases applicable under Section 6.2 using criteria described in the following sections and configuration options from the ICH PSUR Configuration tab -> Summary Tabulations -> PBRER Section 6.2 - Cumulative Summary Tabulations tab for SAEs from clinical trials:
6.3.1 Generating the Case Series for Section 6.2
To generate the case series for Section 6.2, the system:
-
Identifies study cases and serious study cases for the studies that the configured products belong to.
-
Identifies whether a case is serious or not, based on the case level seriousness, primary event seriousness, or a combination of both, as configured in the Argus Console configuration.
Seriousness is configured using System Management (Common Profile Switches) ->Reporting->Periodic->Case Inclusion criteria for the ICH PSUR or CTPR report based on Seriousness).
-
Excludes the following cases from the Section 6.2 case series:
-
Cases whose value matches the non-interventional rules (if any) configured in Section 6.3, if Include Section 6.3 is selected.
-
Cases from non-interventional studies (where REPTYPECODE =M in flexible report type code list) selected in the main inclusion criteria.
-
For each case in the Section 6.2 case series identified, the system prints summary tabulation counts broken down by both System Organ Class (SOC) in the internationally agreed order, followed by preferred terms (PTs) in rows, sorted alphabetically. This is based on the following logic and marked clearly in the temporary tables based on the identified product type:
-
Company or Configured Study Cases
-
Non-Company or Non-Configured Study Cases
6.3.1.1 Company or Configured Study Cases
If the case is being evaluated in a configured study, the system displays all the Section 6.2 case series that are part of study configuration for the product and study being evaluated. Counts are broken down into the following categories:
-
Blinded:
-
If the study and case are both blinded, the system prints the count under Blinded.
-
If the case is blinded (even if study was unblinded), the system prints the count under Blinded.
-
If the case is unblinded (but the study is blinded) and you do not have the privilege to see unblinded data, the system prints the count under Blinded.
-
If you have access to the unblinded data and case is unblinded (but the study is blinded), or case and study both are unblinded, the system shows the counts under their respective categories if you choose to view the unblinded data; otherwise the system prints it under Blinded.
-
-
Investigational Medicinal Product (IMP): If the target product is of one of the suspect products in the study case, the system counts these cases under Investigational Medicinal Product.
-
Active Comparator: If the target product is NOT one of the suspect products in the case, and the product type attribute for the suspect product available in the study is either an IMP or Comparator, the system prints it under the Comparator column.
-
Placebo: If the target product is part of the study but is not one of the suspect products in the case, and the product type attribute for the suspect product available in the case is identified as Placebo in the study configuration, the system counts the case as a Placebo. You can use User Exits to classify different drugs under the Placebo category.
-
No Study Drug Given: This column allows for retrieval of Serious Adverse Events that occurred from the moment the patient signed the informed consent form to the first administration of study medication, where no actual study medication was given to the patient. The system prints any study drug available in the case having product type defined as No Study Drug Given under No Study Drug Given column.
-
Additional Study Drug OR No product Type Configured:
-
If the study configuration does not include the product type for that product, or is marked as Additional Study Drug, the system considers the product type as Investigational Medicinal Product if it is one of the target products and is also a suspect product in the case.
-
If the suspect product in the case is not part of the target product list, the system counts it as a Comparator. You can also use the user exits feature for categorizing products under the Investigational Medicinal Product, Comparator, Blinded, or Placebo columns in the temporary tables.
-
This tabulation also uses the parameter Print Serious Adverse Events or Reactions.
-
Print All Events: The summary tabulation displays all events present in the cases lying within the reporting period. The in period flag is not used. This is the default value.
-
Print only In Period Events: This summary tabulation displays only events added within the reporting period. Events in the cases with Follow-up data that does not fall into the reporting period are not considered.
6.3.1.2 Non-Company or Non-Configured Study Cases
If the case being evaluated is a non-company or non-configured study case, the system prints the suspect products with the product type as Investigational Medicinal Product and follows the rules of Investigational Medical Product and Blinded.
-
The report prints the count for medicinal products in the first column in the report, followed by the Blinded, Active Comparator, and Placebo columns.
-
The report does not suppress the Blinded, Active Comparator, and Placebo columns when there are 0 counts against them. It prints the event count as 0 in each column as long as at least one of the four columns has an event count greater than 0. When PBRER is generated for a product that is a comparator in some studies, and no case qualifies for the report in which that product is a medicinal study drug, the system prints the medical product column with 0 event counts.
-
If the event count is 0 in the Medicinal Product, Blinded, Active Comparator and Placebo columns for a given SOC, the system does not print that SOC in the report.
6.3.2 Grand Totals
The system provides the totals and grand totals in out-of-the-box PBRER tabulations as follows:
-
Subtotal per System Organ Class (SOC) per study medication category (IMP, Blinded, Active Comparator, Placebo, No Study Drug Given).
-
Grand total for all SOCs per study medication category (IMP, Blinded, Active Comparator, Placebo, No Study Drug Given).
6.4 PBRER Section 6.3 - Cumulative and Interval Summary Tabulations from Post-Marketing Data Sources
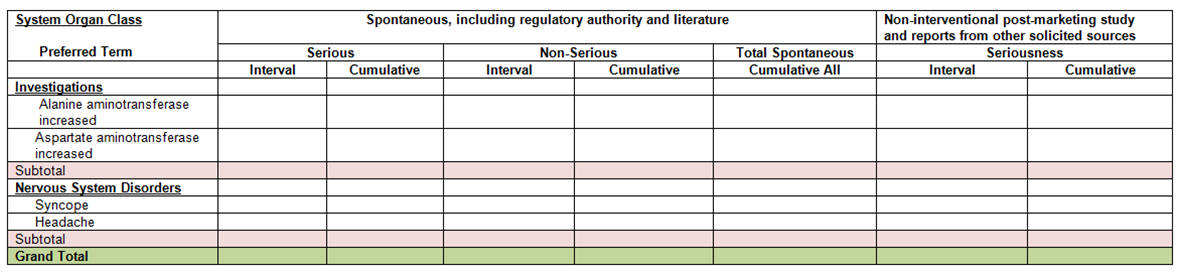
Section 6.3 is a background for the appendix that provides cumulative and interval summary tabulations of adverse reactions—from the IBD to the data lock point of the current PBRER.
These adverse reactions are derived from non-interventional studies and spontaneous ICSRs, including reports from healthcare professionals, consumers, scientific literature, and regulatory authorities. Serious and non-serious reactions are presented in a single table, with interval and cumulative data presented side-by-side.
6.4.1 Case Selection
The system evaluates the list of cases applicable for section 6.3 using the following criteria and configuration options from ICH PSUR Configuration ->Summary Tabulations screen.
The Identify Non-interventional Studies field is mandatory, and you must select at least one of the options if Include Section 6.3 is selected.
If you save the report configuration without selecting a rule or without selecting a Non-Interventional Study report type in the main inclusion criteria, an error message appears.
When you select the Include Section 6.3 option, the system evaluates the list of cases applicable for this section by the following methods and categorizes these cases into the following lists when populating temp tables:
-
Non-Interventional Current cases: Using the main case list identified from the report inclusion criteria, further restricts it to only the Non-Interventional cases based on all rules that are selected, for the Identify Non-Interventional Studies field. This case list is referred to as Non-Interventional current case list.
-
If the Case Classification rule is selected, any case from the main case list qualifies if it has a case classification that matches Identify Non-Interventional Studies.
-
If the Observe Study Type rule is selected, any case from the main case list that has an Observe Study Type qualifies if it has a case classification that matches Identify Non- Interventional Studies.
-
From the main inclusion criteria, any case from the main case list that has a report type that matches a Non-Interventional Study as categorized in the report type flexible re-categorization code list qualifies.
-
-
Non-Interventional Cumulative cases: Using the report inclusion criteria, identifies cumulative Non-Interventional cases for the report corresponding to the configured product and uses the Non-Interventional selected Non-Interventional rule. This case list is referred to as the Non-Interventional Cumulative case list.
-
Spontaneous Current cases: Using the main case list identified from the report Inclusion criteria, identifies the Spontaneous Current case list by restricting it to cases that have a spontaneous report type (where report type code list values do not have the This type includes cases from clinical trials selected).
-
Spontaneous Cumulative cases: Using the report Inclusion criteria, first identifies the Cumulative Main case list, corresponding to the configured product. Using this cumulative main case list, it then identifies the Spontaneous Cumulative case list by restricting it to cases that have a report type in which This type includes cases from clinical trials is not selected.
After the system identifies the four case lists in the temp tables, it prints the cumulative summary tabulation categorized into the Spontaneous, and Non-interventional columns:
-
The Spontaneous column has two sub-columns based on seriousness of the event: Serious, and Non-serious.
-
Both the Serious and Non-Serious columns under Spontaneous have two sub-columns: Interval, and Cumulative.
-
The Non-interventional column has a sub-column, Serious, based on events that are serious.
-
The Serious column under Non-Interventional has two sub-columns: Interval, and Cumulative.
The system prints the summary tabulation counts broken down by both System Organ Class (SOC) sorted in the international order, followed by Preferred Terms (PTs) in rows, sorted alphabetically. The report prints SOCs with zero counts if there are no events reported.
It uses the Spontaneous Current case list for determining the Interval counts under the Spontaneous column (stratified based on Seriousness) and the Spontaneous Cumulative case list for the Cumulative counts under the Spontaneous column (stratified based on Seriousness).
The system uses the Non-Interventional Current case list for the Interval counts under the Non-Interventional column (stratified based on Seriousness) and the Non-Interventional Cumulative case list for the Cumulative counts under the Non-Interventional column (stratified based on Seriousness).
The summary tabulation prints the Total Spontaneous Cumulative all sub totals for Spontaneous column as total of Spontaneous Cumulative Serious plus Spontaneous Cumulative Non-Serious counts.
This tabulation also uses the parameter Print Serious Adverse Events or Reactions.
-
Print All Events: The summary tabulation displays all events present in the cases lying within the reporting period. The in period flag is not used. This is the default value.
-
Print only In Period Events: This summary tabulation displays only events added within the reporting period. Events in the cases with Follow-up data that does not fall into the reporting period are not considered.
6.5 Sample Report
The following sections discuss the parts of the report.
6.5.1 Cover Page
All BI Publisher reports have a cover page with information about all report parameters and their values used in the report.
Figure 6-4 represents a sample PBRER cover page:
6.5.1.1 Information on Drugs and Studies
After the list of report parameters, the cover page provides information about the list of products or study licenses selected in the corresponding Argus Safety Configuration screen for the current report run. The following format is used for this information:
6.5.1.1.1 List of Configured Drugs and Licenses:
-
Product Name 1 or License 1
-
Product Name 2 or License 2
If you select a study on the CTPR Report Configuration screen, the system displays the list of licenses available in that study for populating this section.
List of Case Numbers
The system also prints all case numbers that are part of current report data. These are displayed after the list of products and licenses.
After the list of case numbers, all modifications made to the case series are printed in the reports in the order they were performed.
6.5.2 Labels Configured for Drugs in the Drug List Format
The following format is used for displaying this information in the QA section:
Here:
-
Drug Name prints the name of the product configured for running the report.
-
Label Name prints the datasheet name for the corresponding product or product family.
-
Label Version prints the revision number of the corresponding datasheet.
-
Effective Date prints the activation date of the corresponding datasheet.
If any of the above fields is null, the field is blank.
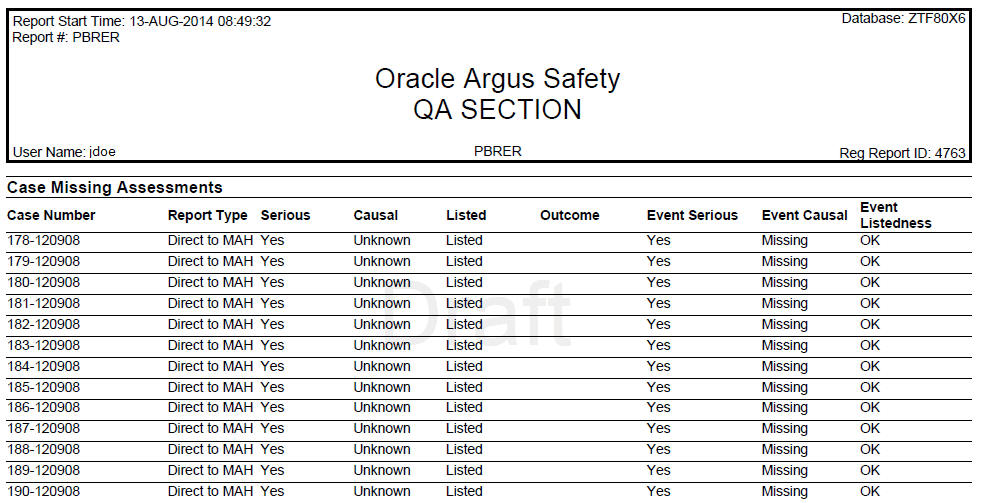
6.5.3 Cases with Missing Assessments
In this sub-report, the system displays cases that are included in the PBRER, but one or more of the following have not been assessed:
-
Case Seriousness
-
Report Type
-
Case Causality
-
Case Listedness
-
Case Outcome
-
Event Seriousness
-
Event Causality
-
Event Listedness
Case Seriousness, Case Causality, Case Listedness, Case Outcome, and Event Seriousness print their corresponding values, while Event Causality prints OK if the information is present and Missing if missing. Case seriousness and Event seriousness print values are Unknown if seriousness is undefined. The system prints event listedness OK if listed or unlisted. Otherwise, it prints it as Unknown.
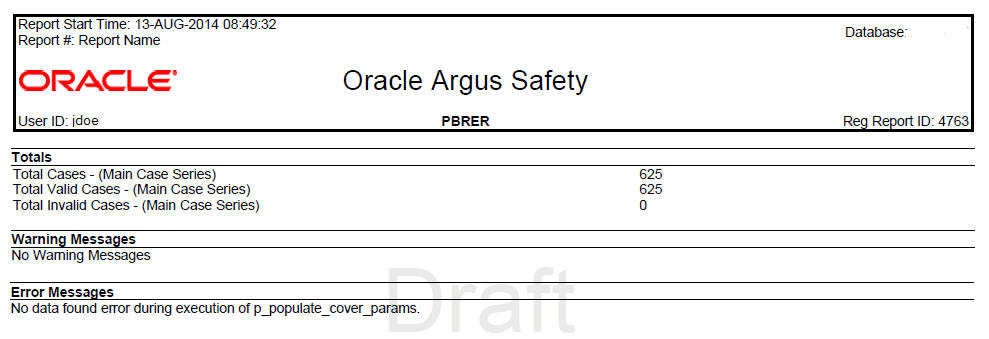
6.5.4 Trailer Section
The following information is displayed in the trailer section of PBRER:
Table 6-1 Trailer Section Information
|
Total Cases-(Main case series) |
Count of cases that are part of the main case series. |
|
Total Valid Cases-(Main case series) |
Count of cases that are part of the main case series and also fall into the reporting interval. If there are no valid cases, 0 is printed in the count. |
|
Total Invalid Cases -(Main case series) |
Report prints the count of cases which are part of the main case series but do not fall into the reporting interval. If there are no invalid cases, 0 is printed in the count. |
|
Warning Messages |
All warning messages related to case series and report output. This section prints the exact warning generated during report execution. If there is no warning, the system prints No Warning Messages. |
|
Error Messages |
All error messages related to case series and report output. This section prints the exact error message generated during report execution. If there is no error, the system prints No Error Messages. |
The following format is used for displaying the information:
6.6 Executing the PBRER from BIP
To execute a report from BIP, perform the following steps:
-
Sign in to BI Publisher, and click Catalog.
-
Select the tree entry for a report and select Reports.
Figure 6-9 The Reports Tree Entry Screen
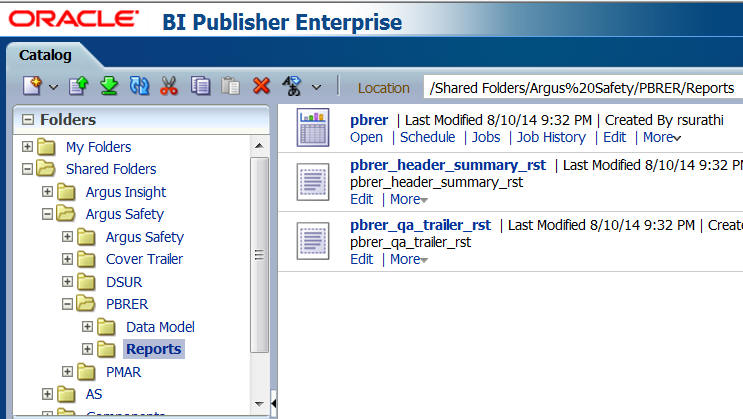
Description of ''Figure 6-9 The Reports Tree Entry Screen''
-
The available reports are displayed on the screen. Click the Open link for a report.
-
The report screen appears. Click the Open tab.
-
Select the required values from the parameter drop-down lists.
-
Select a format. This executes the report.