8 Manage Your Periodic Report Submissions
Periodic reporting plays a key role in the safety assessment of drugs. It involves the compilation of safety data for a drug over a prolonged period of time (months or years), as opposed to single-case reporting which, by definition, involves only individual AE reports. The advantage of Periodic reporting is that it provides a broader view of the safety profile of a drug.
Prepare Content for Periodic Reports
Prepare your Aggregate Reports
These reports are executed in the background through the AG Services. A new argus service named Batch Aggregate Report Generation is available for this purpose.
Understand Aggregate Reports
-
The date and time printed on the following reports are the date and time the query is executed for case qualification. They are not the date/time the query was completed and the report obtained Web Server.
-
Case Listing Report
-
Case Data Analysis Report
-
-
The system converts the following elements that display in the case form as actual text on the Case Listing and CIOMS II Line Listing reports:
-
Duration of Administration
-
Time Between First Dose/Primary Event
-
Time between Last Dose/Primary Event
-
-
Capture the report follow up number in the Regulatory Reports section. The system prints the report follow-up number on the expedited reports in the following format:
F/U# X
where:
X is the report follow-up number.
-
For an initial report, the system prints initial in the column.
-
If there are no reports for the case in the Case Listing/CIOMS II Line Listing, the column is left blank.
-
This option is available on the CIOMS II Line Listing/Case Listing reports.
-
The system uses the IE offset of the client workstation to print the date and time component for all system-calculated fields on the Case Listing/CIOMS II Line Listing reports.
-
You can filter cases in the following aggregate reports based on the lock/archived date.
-
When you select Case Locked/Archived date, the system limits the cases based on whether the case is locked/archived within the specified time frame.
-
The lock date is considered the locked date.
-
If you select the Case Patient or Reporter Information in the Case Listing or CIOMS II Line Listing reports and the Protect Confidentiality field is checked for the patient or reporter Information, the system does not print the relevant patient or reporter Information selected in the Case Listing or CIOMS II Line Listing report.
-
If you do not have permission to view the reporter or patient information, the corresponding reporter or patient elements selected on the Case Listing or CIOMS II Line Listing report are blank.
View Saved Aggregate Reports
The System Reports Library displays the list of the saved aggregate reports including Case Data Analysis, Case Listing and CIOMS II Line listing reports. This screen displays only the reports either created by the logged in user or the reports that are shared for your user group during report creation.
-
The Aggregate report supports only PDF or CSV formats.
-
The system report library screen, provides the way to filter the reports through:
-
Report Name
-
Description
-
Report Type
-
Output Type
-
Author
-
Last Modified
-
Available for Periodic
-
Shared
-
-
The reports can be executed through the Print option. When you click Print, the applications opens the Report Batch Printing dialog box, where you can run or schedule the report for the desired format (PDF/CSV). You can send an email for the completion status.
-
The open option allows to open and edit the memorized reports.
-
If the user clicks Transmit, the system generates the selected report and starts the transmit process. The system displays the Recipient pop-up.
Note:
A shared report can only be deleted by the Administrator or the user who created it.Create a Case Data Analysis Report
The Case Data Analysis Report enables you to view quantities of cases over time in a Cross-Tabular Fashion.
-
Select Reports > Aggregate Reports > Case Data Analysis.
-
In the Case Data Analysis Report view, select the information that must appear in the report.
-
In Row1, select the field the system uses to group cases by row.
-
In Column1, select the data the system uses to group cases by column.
-
In Row2, select the field by which each Row1 item will be categorized.
-
In Column2, select the field by which each Column1 item will be categorized.
-
Select a product family to which the report applies, if appropriate.
-
In Selection for Row1, select the value for row 1 by which the report must be restricted.
-
Specify an advanced condition, as appropriate.
-
Select Report Number of Cases or Report Number of Events, depending on the number of cases or the number of events to be entered in the report.
-
If you select Report Number of Events, you can specify the kind of events (serious listed, non-serious listed, serious non-listed, or non- serious non-listed) that will appear in the report.
-
Select whether only the top few items should be displayed and enter the number of items that should be displayed.
-
Check the Show% of Total checkbox to specify the percentage in each cell in the report.
-
Check the Blinded checkbox to hide blinded information in the report. This field is unchecked and disabled for a restricted user.
-
Check the Use Case Search Results checkbox to limit the Case Data Analysis only to the cases present in the Case Search dialog box.
-
Specify a date range for the cases that will appear in the report.
-
Enter a title for the report.
-
Click the Share this report with other users if there is a need to share the report/report output to other users. Note that the report may contain sensitive data. By default, the report is shared with all users. You can restrict it through Group, where the allowed user groups can be selected. When a report is shared for a user group, only members of that group and the report creator can access the report further.
-
The report can be used in the periodic report using Make available for use in Periodic Reports.
-
The Case Data Analysis report, in addition to the PDF and CSV format, supports the chart format too.
This report output can be selected via Report Display Type field. The following chart types are available:
-
Bar Graph
-
Line Chart
-
Area
-
Pie
-
Donut
-
Stacked
Note:
The chart reports are run in sync and available only the user's browser and not stored in the database.
-
-
Click Print to execute the report in background. This report can later be viewed under system reports library.
-
The memorize button saves the report in the application. It does not execute the report.
Create a CIOMS II Line Listing Report
The CIOMS II line listing report is a common format desired by Drug Safety professionals for reviewing cases. Create this report from the CIOMS II Line Listing dialog box.
-
Select Reports ->Aggregate Reports -> CIOMS II Line Listing.
-
On the CIOMS II Line Listing Criteria tab, select information for the header, footer, product family, advanced condition (if any), cases to include, and date.
-
Select either the Case Creation Date or Case Receipt Date radio button and specify a date range to run the report.
Note:
If you perform a search and return a list of cases to the Case Search screen, the Use Case Search Results is visible. Checking this box will disable all selection criteria with the exception of Include Unlocked Cases. For example, the Advanced Condition and Date Range will be disabled. -
In the Line Listing tab, add or remove the appropriate fields.
-
In the Grouping tab, add or remove elements, insert a page break and change the sort order (if desired).
-
Click Memorize to open the Memorized Report dialog box.
The Memorize button saves the report in the application. It does not execute the report.
-
Click Print to execute the report in background. This report can later be viewed under system reports library.
-
This report can be shared with other users of Argus and the sharing can be restricted through the User Group selection.
Create a Case Listing Report
The Case Listing Report enables you to filter cases based on Case Initial Receipt Date and Case Creation Date. You can select multiple entities from the List of available fields using the CTRL+CLICK functionality.
-
Select Reports -> Aggregate Reports -> Case Listing.
-
When the system opens the in the Case Listing Reports view select the information to appear on the report.
-
Select the fields that are to appear in the report from the Available Fields list.
-
Click Add. Repeat this process for each field that must appear in the report.
-
Use Move Up and Move Down to arrange the fields in the Selected Fields list.
-
Check the Blinded checkbox to hide blinded information in the report.
-
Specify an Advanced Condition, if appropriate.
-
Specify a date range for the cases to be displayed in the report.
-
If you check the Include in Header checkbox, the selected date range is displayed on the report.
-
Under Sorting Order, select the fields by which the cases will be sorted. You cannot sort the cases by fields that do not appear on the report.
-
Enter the title of the report.
-
Click Memorize to memorize the criteria specified for a particular report.
The Memorized Report dialog box appears.
-
Save, Delete or Cancel the report, as applicable.
-
Click OK in the Case Listing Reports screen to generate the report. The report will be generated in PDF format.
Periodic Report Types
On the Reports menu, hover over the Periodic Reports option, to go to any of the Periodic Reports.
You can create the following periodic reports:
-
Clinical Trial Periodic Reports
-
ICH PSUR Reports
-
US IND Periodic Reports
-
US NDA Periodic Reports
Store Periodic Reports in Documentum
When you approve an expedited report from within Argus, the system exports the report as a PDF file and saves it in the Documentum database by Argus Safety Service. From this point, you can perform document reviews within the Documentum system.
When the report is ready to be submitted, mark the report as Submitted from within Argus. Argus Safety Service updates the status of the report within Documentum to Submitted.
If the report is to be transmitted via fax or email, Argus Safety Service marks the report as a successful submission in Documentum only after the fax or email transmission has succeeded.
View a Summary of Periodic Regulatory Reports
You can select any of the following options in viewing the summary of a periodic regulatory report:
-
To view the regulatory reports for a particular case (scheduled, generated, and submitted), open the Regulatory Reports tab of the Case Form.
-
To view all scheduled, generated, and approved reports, as well as other outstanding action items assigned to you or your user group, select Reports in the Worklist menu.
-
To view a list of all scheduled, generated, and approved periodic reports, select Periodic Reports from the Reports | Compliance menu.
Use the Library Page
The Periodic report has a library page from where you can create a new report, execute an already created report, view the output and so on.
-
PSUR (legacy PBRER, PSUR and Flexible PBRER, PMAR reports)
-
CTPR (legacy CTPR and Flexible DSUR reports)
All fields that are used by the Flexible DSUR is highlighted with blue BIP icon.
-
IND
-
NDA
While a report is running, it can be modified or run again until the previous run is complete.
Prepare Content for a Clinical Trial Periodic Report
The Clinical Trial Periodic Reports (CTPR) are created to report the IND Annual reports and EU Clinical Trial Directive line listing reports to FDA.
Create a Clinical Trial Periodic Report
-
Select Reports > Periodic > CTPR (Clinical Trial Periodic Reports) to open a list of CTPR Reports.
-
To create a new report from an existing report in the list, click Copy or Modify.
-
Click New Report to create an entirely new report. The Clinical Trial Periodic Reports pop-up window opens.
-
Enter an appropriate name for the report under Report Name.
-
Use the tabs in this pop-up window to configure the CTPR.
Enter Common Fields Information
The Report Name, Report Category and Report Sub-Category fields are common to all tabs of the Reports.
| Field | Description |
|---|---|
| Report Name | Enter a name for the Report. The name entered here is displayed in the Reports menu. |
| Report Category |
|
| Report Sub Category |
|
Configure Subject in the Report Header
The Subject of Report tab is used to configure the report header and to specify the agency, products, etc. for which the CTPR is applicable.
| Field | Description |
|---|---|
| Available Reporting Destination | Displays the list of configured Regulatory Agencies. Select an agency from the list displayed in Reporting Destination and click Add to add the report to the Selected Destination list.
Select multiple agencies by holding the CTRL key when you click them. Likewise, select an agency from the Selected Destination list and click Remove to remove it from the selected destination. |
| Primary Agency | Select the primary agency for the report.
When you submit a Periodic report, it goes to the selected Primary Agency. |
| Selected Reporting Destination | Displays the list of agencies where the report is being sent. Select an agency from the list displayed in Reporting Destination and click Add to add the report to the Selected Destination list.
Select multiple agencies by holding the CTRL key when you click them. Likewise, select an agency from the Selected Destination list and click Remove to prevent it from being sent to the selected destination. |
| Ingredient | Automatically displays the Ingredient as provided in the Subject of Report dialog box.
You can choose whether to view this field or not. Click the checkbox displayed with this field to hide or view it. |
| Trade Name | Automatically displays the Trade Name(s). Multiple trade names are also displayed together, separated by commas.
You can choose whether to view this field or not. Check the checkbox displayed with this field to hide or view it. |
| International Birth Date | Automatically displays the earliest license awarded date, when a user selects an Ingredient and a Product.
You can choose whether to view this field or not. Check the checkbox displayed with this field to hide or view it. |
| Print all configuration criteria on separate cover page | Mark this box to print out the configuration of this report when the report is printed. This is only available when PDF option is selected during printing. |
| Print page numbers on reports | When checked, this option enables the user to print page numbers on a periodic report. This is the default for all report configurations.
If this checkbox is not checked, the following occur.
|
| Allow access to report cases through Case Series | When the report is run as final, it creates a Hit List, which can be retrieved from other areas of the application where advanced conditions can be selected. Click this checkbox to report cases through the Hit List. |
Select Products to include in CTPR
The Product Selection tab enables you to select the products included in the CTPR report.
| Field | Description |
|---|---|
| Available Ingredients | Displays the list containing the Ingredients used for the product configuration. Select an ingredient from the list displayed in Available Ingredients and click Add/Remove to add/remove the ingredient.
You can select multiple ingredients at a time. |
| Filter | Enter an Ingredient name and click Filter to search for the entered ingredient within the Available list of Ingredients. |
| Selected Ingredients | Displays the list of ingredients selected from the Available Ingredients list. |
| Available Countries | This list is auto-populated and displays only the countries with a license containing the ingredient selected from the Available Ingredients list. |
| Selected Countries | Displays the countries selected from the Available Countries list. |
| Indication | This list contains the Indication configured for the product containing the ingredients in the Available Ingredients section. The selections made from this list get displayed in the Available Products section.
You can select multiple Indications from the list at a time by pressing the CTRL key and clicking the different Indication entities. |
| Formulation | This list contains the Formulation configured for the product containing the ingredients in the Available Ingredients section. The selections made from this list get displayed in the Selected Products section.
You can select multiple Formulations from the list at a time by pressing the CTRL key and clicking the different Indication entities. |
Configure License or Study in the Report Header
The License/Study tab is used to configure the report header and to specify the agency, products, etc. for which the CTPR will be applicable.
| Field | Description |
| Available Licenses | Contains licenses that use the ingredient selected in the Ingredient field. This field is automatically populated when an ingredient has been selected. |
| Selected Licenses | Displays the licenses selected from the Available Licenses list by clicking Add/Add All. You can select Licenses that are related to different ingredients for a report. |
| Available Studies | Contains studies that use the ingredient selected in the Ingredient field. This field is automatically populated when an ingredient has been selected. |
| Selected Studies | Displays the studies selected in the Available Studies list by clicking Add/Add All. You can select studies that are related to different ingredients for a report. |
Select Inclusion Criteria
The Inclusion Criteria tab allows you to select search parameters for inclusion of cases in a periodic report.
The top section of the dialog box allows you to specify the type of cases that will be included in the periodic report.
| Field | Description |
|---|---|
| Case Creation Date | Allows you to specify a range of cases by the date when the case was created. |
| Case Receipt Date | Allows you to specify a range of cases by the initial receipt date.
Note: The Date Range is only available when an unscheduled CTPR is being created. You must specify only one date range out of Case Creation Date and Case Receipt Date. |
| Case Locked/Archived Date | Allows you to specify the date when the case was locked/archived. |
| Use Current Version | Allows you to use the latest revision to populate the data within the selected reports. |
| Use DLP Version | Allows you to use the case data of the version as of the specified DLP Version. |
| Age Groups | Allows you to include or exclude cases based on the patient's age group. Select all the age group categories that apply. |
| Restrict Cases to countries where studies are active | References study configuration to determine if the case was submitted during the dates the study was active.
Note: This option is available only if a study is selected from the Available Studies section in the License/Study tab. It should not be used for Centrally approved products (CAP), which only have an EU license. |
| Advanced Condition | Allows you to specify an advanced condition that must be satisfied by each case that is included in the report. Ensure that the advanced condition or the advanced condition query set that is specified here does not contradict any other criteria specified in the dialog box. |
| Datasheet for Listedness | Select the Datasheet to look against for Listedness when running the report.
Note: Select the ALL datasheet to use the most conservative listedness for the primary event, or the Case Listedness for the tabulation. |
| Use Assessment in Cases | When selected, the CTPR Report will use the Case Event Assessment when performing datasheet listedness calculations. |
| Re-assess cases against datasheet in effect at the beginning | When selected, the CTPR will re-assess the cases in the line listing based on the Active Datasheet on or before the Start Date of the Reporting period. |
| Re-assess cases against datasheet in effect at end | When selected, the CTPR will re-assess the case in the line listing based on the Active Datasheets on or closest to the end date of the CTPR Reporting end date range without exceeding that date. |
| Reference Type (Clinical Reference Data Element) | Select the reference type to be displayed on the Main Listing if Clinical Study Reference is selected as a Data Element in the Available Data Elements section of the Line Listing tab. |
| Expeditable Only | This option is enabled only when an agency has been specified on the Subject of Report tab. Check this option to include only those cases that have submitted expedited reports to the specified Primary Agency. |
| Exclude Follow-up cases | Follow-up cases are cases with a significant follow-up in the Clinical Trial reporting period where the initial receipt date is in a prior period. Check this option to exclude follow-up cases from appearing on the Clinical Trial report. |
| Include Unlocked Cases | Check this option to allow cases that have not been locked for reporting to appear on the report. |
| Use Datasheet Assessment for UDF Tabulations | Allows you to select datasheet for a report to make UDF tabulations.If no datasheet is selected, the most conservative listedness is chosen, i.e. Unlisted followed by Listed. |
| Add Cases not included in previous reporting period | Allows you to add cases which were not included in the previous reporting period.
You can enter the start date of the period in the Start Date field. |
Use the Data Lock Point (DLP) Version
DLP primarily uses two processes:
-
Last Completed Version - This option always uses the case version with the last lock that existed prior to the DLP or As of reporting date. This option does not enable data cleaning
DLP saves only the last revision when multiple saves are performed in the same job session.
-
Next Completed Version - This option uses the current case lock or the next following case lock if the case version was initiated prior to the DLP or the As of reporting date with two exceptions:
-
If there is no case lock for the current version that was received prior to the DLP, the last (current) case revision is used.
-
If there is a case version after the case lock that was created for the purpose of data cleaning, it is to be used instead of the first locked case revision.
-
If there are multiple contiguous case versions following the first case lock for the purpose of data cleaning, the last case data cleaning version is used.
Note:
The Data Cleaning option is available only with the DLP option Use Next Completed Version (Includes Data Cleaning).
-
Use DLP Queries for Dates
You can perform DLP queries for the following:
-
Receipt Date -- date entered by the user during case creation
-
Initial/Follow up Receipt Date
-
Safety/Safety Follow up Receipt Date
-
System Date (Case Creation Date) - date the case was physically entered
Use As of Reporting Function
The as of reporting function returns the same case version results as the DLP Options, with the only difference that the date depends on the As of date instead of a DLP date.
Find DLP and As of query functionality
The DLP and as of query functionality is available in the following application modules:
-
Periodic Reports
-
PSUR including all User defined Tabulations and expedited reports within the Periodic Report
-
CTPR including all User defined Tabulations and expedited reports within the Periodic Report
-
IND including all User defined Tabulations
-
NDA including all User defined Tabulations and expedited reports within the Periodic Report
-
-
System Reports
-
CIOMS II Line Listing
-
CDA Reports
-
Case Listing Reports
-
Include Line Listing
| Field | Description |
|---|---|
| Include Line Listing | Allows you to select whether you want the Line Listing Data Elements printed with the CTPR Report. |
| Blind Line Listing and Summary Tabulations | Hides the selected listings from being displayed |
Add Data Elements
Select the checkbox associated with a data element (field) to add that field to the report.
-
By default, the system print s unavailable fields on the report, and they cannot be changed.
-
Required data elements are printed as columns in the report. The optional data elements are printed as separate rows below the column data for each case.
View Selected Data Elements
This section lists the selected elements and enables you to arrange the order in which these are to be printed. Click the Up or Down buttons to arrange the listed elements above or below in order of priority.
| Field | Description |
|---|---|
| MedDRA Hierarchy from Cases/Dictionary | Select Cases to populate the data from the case data. Select Dictionary to populate the data from the MedDRA dictionary. |
| Print Only the Term (Preferred Term or Lower Level Term) | Prints only the event Preferred Term (PT) or event Lower Level Term (LLT) as per the selected radio button. Select the PT option to print only the preferred term and not the verbatim description. |
| Print Dose Text in place of regimen dose | Prints the dosage and frequency information from Dose Description field instead of Regimen Dose. |
| Indicate if case was expedited previously | This checkbox is selected if a primary agency has been selected in the Subject of Report tab. Cases for which an expedited report was previously submitted to the selected authority are marked with an asterisk. |
| Local Language | Allows a user to specify which Local language for a multi-language field is to be printed i.e. the Abbreviated Narrative field. |
| Print event info (Serious, Un-listed, Related) as Column | Select this checkbox to print the Seriousness, Listedness and Causality under the Event Verbatim column.
Note: Events having listedness of Unknown are considered Unlisted. If only diagnoses are assessed for event assessment, the events which are associated with a Diagnosis but have been marked with Diagnosis as Np display - for both listedness and causality. |
| List cases only once, under the primary event | Select this option to view the details of cases in the Main Line Listing only once under the Primary Event |
| List cases under all events, details under the primary event | Select this option to view the details of cases in the Main Line Listing only once under the Primary Event, while non-primary events are listed under their respective event hierarchy with a reference to the primary event body system. Therefore, use this option when grouping on Main Line Listing is by the Event Body System. |
| Print Product Indication for the product selected in the report | Select this option to print Product Indication for the product selected in the report. |
Group Cases
| Field | Description |
|---|---|
| Available Groupings | Allows a user to group cases together from the given list. Select the desired groupings from the list and click Add to move the grouping to the Selected Groupings list. Up to 10 grouping options can be selected. |
| Selected Groupings | Lists the added groupings made available from the Available Groupings list, and reports the groups in the order they were selected. |
| Ascending | Select this checkbox to sort the selected entities in ascending order. |
| Page Break | Select this checkbox to start the cases from a new page, while also keeping the sorting together for every selected page break. |
| Available Sortings | Allows a user to sort cases together from the given list. Select the desired sortings from the list and click Add to move the sorting to the Selected Sortings list. |
| Selected Sortings | Allows a user to further sort cases without a total count for each sorted item. Up to 3 levels of sorting can be selected from the Available Sortings list. |
Specify Summary Tabulations for Line Listing
The Summary Tabulations tab enables you to specify which summary tabulations/Listings will appear along with the line listing.
| Field | Description |
|---|---|
| Include Index of Cases in CTPR | Create an index page of case numbers, for all cases included in the CTPR. |
| Include Summary of Cases Missing Assessments | This option creates a sub-report of cases missing one of the following items:
Click this checkbox to create one or both of the following sub reports: Cases Missing Assessments - This sub-report displays cases that have been included in the CTPR line listing, but one or more of the following have not been assessed:
Cases Not Included in Report - This sub-report displays cases that have not been included in the CTPR line listing as a result of missing one or more of the following items:
|
| Count of Cases per Report Type | This option prints a Sub Report that counts the number of cases versus the Report Type, based on the cases within the CTPR.
The Cases per Report Type can be either of the following: Count Cases with Initial Expedited Reports: Counts cases with initial Expedited report. Count of cases with Follow-up Expedited report: Counts cases with Follow-up Expedited report. Total Count of Initial Cases in the Report: Counts any (serious - non-serious) cases received during the reporting period. Total Count of Follow-up Cases in the Report: Counts any (serious - non-serious) follow-up cases entered during the reporting period. Cumulative Count: Count of cases received from the start of the trial. |
| Event Count per Study Drug | Creates a sub-report with Event count per Study Drug based on the selected causality. 2 configurations are possible as to allow for a count of related events vs. non-related events. |
| All Study Drugs in Single table | Suppresses 0 current columns (with their cumulative) and print everything in a single cross tab. |
| Grouped by Study Drug | Prints a cross tab report for every product. Prints the cumulative totals even if the current period has no events. |
| Event Type to Include | Prints SUSAR events on the CTPR Report based on the option selected from the drop-down list:
|
| Include Line Listing Tabulation | Select this checkbox to view a pre-defined summary tabulation of Report type, Seriousness and Listedness of all cases in the CTPR. |
| Include Initial Cases | Select this checkbox to include initial cases in the CTPR tabulation. |
| Additional Expedited Report Forms (CIOMS/MedWatch/VAERS) | Allows you to print CIOMS/MedWatch/AERS forms (as per selection) for its respective regulatory agency (as selection), for all cases in the report. It also provides an option to have (or not have) a watermark in the forms. |
CIOMS Reports—Fields Descriptions
| Field | Description |
|---|---|
| Print CIOMS reports for serious/unlisted cases | Allows a user to print CIOMS I forms for all Serious/Unlisted (Case Level) cases appearing in the CTPR.
CIOMS contain Internal or Other text printed on them when the CTPR is printed using the Internal or Other option. |
| Include Periodic Numbering on the CIOMS reports | Numbers the requested CIOMS I with a periodic format. (i.e. A-1-1 of 2, A-1-2 of 2, A-2-1 of 1, A-3-1 of 1 etc.). The index is modified to also contain the Periodic paging of each CIOMS report. |
Cumulative Summary—Fields Descriptions
| Field | Description |
|---|---|
| Include Cumulative Summary | Select the checkbox to create a sub-report count of events, grouped by Product and Body System (SOC) and sorted by Preferred Term. The sub-report contains a previous date range count of events (comparative date range), a current date range count (current CTPR date range) and a cumulative count (all dates) of events assessed against the product(s) of the CTPR and matching the inclusion criteria. |
| Comparative Date Range | Allows a user to specify the previous date range as a comparison date for the events counted, and therefore should not overlap with the current date range specified on the CTPR inclusion criteria tab. |
FDA CTPR Support—Fields Descriptions
| Field | Description |
|---|---|
| FDA CTPR Support Section | |
| Include Adverse Event Summary | Select this option to generate a sub-report of events from the line listing. This sub report is grouped by Body System and Preferred Term.
Note: This section can be used if the company has obtained an FDA waiver to submit a CTPR instead of an NDA report. |
| Causality | Select the desired causality from the list.
Ignore - Counts events regardless of causality assessment. Causal - Counts events where the causality is considered reportable in the Causality Category configuration in List Maintenance. Not Causal - Counts events where the causality is considered non-reportable in the Causality Category configuration in List Maintenance. As Determined - Counts events where As Determined causality meets the above selected causality criteria. As Reported - Counts events where As Reported causality meets the above selected causality criteria. Both - Counts events where both As Reported and As Determined causality meet the above causality criteria. Either - Counts events where either the As Reported or As Determined causality meets the causality criteria. |
| Only Cases with HCP Reporter | Select this checkbox to include events for only those cases that feature an HCP reporter |
| Diagnosis | Select this radio button to ensure that only events marked as diagnosis are counted. |
| Diagnosis & Symptoms | Select this option to ensure that all events are counted in the sub-report. |
| Separate Diagnosis & Symptoms | Select this option to include all SUSAR events in the CTR Report. |
| Print Unsubmitted | This option allows a user to print MedWatch or VAERS forms for U.S. cases. The following types of cases will be excluded:
|
| Exclude Reports that are Non-Serious and Listed | Allows a user to suppress MedWatch or VAERS forms from printing for Non-Serious listed cases where all events are non-serious and listed for the datasheet specified. |
| Use Periodic numbering on the Reports | Numbers the requested forms with a periodic format. (i.e. Periodic Page 1 - 1, Periodic Page 1 - 2, Periodic Page 2 - 1, Periodic Page 2 - 2, etc.) An index with the Case Number is also included. |
Generate Single Case Submission Report
| Field | Description |
|---|---|
| Single Case Submission Support Section | |
| Generate Periodic ICSR Submissions for any cases in this Periodic Report that does not have at least one scheduled single-case report during the reporting period to the following Reporting Destination(s): Modify | Select this checkbox to generate the ICSR Reports only for the cases, where a Periodic ICSR Report for the message type chosen, does not exist.
Select one or more trading partners from the list box. Important: Any case that does not have an expedited or single case periodic submission to a trading partner, must have an ICSR report scheduled as a part of the Periodic submission. Click Modify to select a different Reporting Destination. |
| Schedule these single-case Periodic Reports to the following Reporting Destination | Select a single-destination trading partner for Periodic Reports from the drop-down list box. |
| Using the Message Type | Select the required message type from the drop-down list box |
Select Summary Listing
The UD Summaries tab allows you to specify which summary listings will appear along with the line listing.
| Field | Description |
|---|---|
| Include these summary tabulations/listings based on the set of cases presented in the line listing | Allows you to select from pre-configured summary tabulations/listings based on Case Data Analysis, Case listing or CIOMS II line listing reports. These tabulations are based only on the data included in the line listing. Select the Exclude Follow-up Cases checkbox to filter out follow-up cases from the attached report.
If the Exclude Follow-up Cases option is selected on the Inclusion criteria tab, this option is ignored and follow-up cases are always filtered out. |
| Include these summary tabulations based on all cases | Allows for additional sub-reports based on the 'Case Data Analysis' template, to be included as an output for the all cases in the database that meet the CTPR inclusion criterion for all dates. |
| Include these summary tabulations/listings based on the Date Range | This option allows for additional sub-reports based on Case Data Analysis, Case listing or CIOMS II line listing reports to be included as an output for the cases meeting the CTPR inclusion criterion for the Date Range specified when adding the sub-report.
The dates are based on either the Case Creation Date or the Initial Receipt Date as entered on the CTPR Inclusion Criteria tab. Check the checkbox to the right of the sub-report to ignore considering follow-up cases for the sub-report. |
Schedule the Report
The Scheduling tab enables you to specify details of how often the periodic report will be scheduled.
| Field | Description |
|---|---|
| Start Date | This is the International Birth Date for the PSUR product. This date is computed as the earliest Awarded date for any license of any type. |
| Recalculate | Allows a user to recompute the International Birth Date of the PSUR Product. This date can be overwritten/manually entered, if needed. |
| Report is due xx days after selected end date (creation or receipt date) | Enter the number of days when the report will be due after the end date specified for the scheduling period. |
| Automatically generate report xx days before/after selected end date at xx:xx | Allows a user to specify the timing of the automatic report generation, by specifying the number of days before/after the selected end date of the report. |
| Group | Allows the user to select the group to which the automatically generated report is to be assigned |
Setup Frequency of the Scheduled Reports
| Field | Description |
|---|---|
| Frequency | Allows a user to specify the interval required for this scheduling period. |
Configure Security Level of the Report
The Security tab is used to configure the security level for the PSUR.
| Field | Description |
|---|---|
| Share this Report with Other Users | Click this checkbox to share this report with other users. Specify the privileges to be granted to groups by adding the group name from the Users Groups list to either the Execute or Modify and Execute list. A user group can exist in only one of these access lists. |
| User Groups | The groups listed here have no access to the PSUR report template. Click Add or Remove to move them to another access list. |
| Execute | The groups listed here have read and execute access to the shared PSUR report template. |
| Modify & Execute | The groups listed here have read, execute and modify access to the shared PSUR report template. |
Prepare Content for an ICH PSUR
The Periodic Safety Update Reports (PSURs) are created on a periodic basis to enable regulatory authorities to monitor the safety of a marketed product. This information is used to view new data about the product acquired from appropriate sources. It helps relate this data to the patient exposure and also indicates whether changes should be made to the product information in order to optimize the use of the product. Requirements on the due date of periodic reports may differ for different regulatory authorities.
Create Periodic Safety Update Reports (PSURs)
-
Select Reports > Periodic > ICH PSUR Reports. A list of PSUR Reports opens in the right frame.
-
Click Copy or Modify to create a new reports from an existing report.
OR
Click New Report to create an entirely new report.
-
When the system opens the ICH PSUR Line Listing Reports dialog box, enter an appropriate report name in the Report Name field.
-
Use the tabs to configure the PSUR.
Configure Subject in the Report Header
The Subject of Report tab is used to configure the report header and to specify the agency, products, etc. for which the PSUR will be applicable.
| Field | Description |
|---|---|
| Primary Agency | Select the Primary Agency. |
| Reporting Destination | Displays the list of agencies from where the report is being sent. Select an agency from the list displayed in Reporting Destination and click Add to add the report to the Selected Destination list.
You can select multiple agencies from the list of agencies such that the report can be submitted to multiple agencies at the same time. Likewise, select a report from the Selected Destination list and click Remove to prevent it from being sent to the selected destination. |
| Selected Destination | Displays the list of agencies where the report is being sent. Select an agency from the list displayed in Reporting Destination and click Add to add the report to the Selected Destination list.
You can select multiple agencies from the list of agencies such that the report can be submitted to multiple agencies at the same time. Likewise, select a report from the Selected Destination list and click Remove to prevent it from being sent to the selected destination. |
| Ingredient | Automatically displays the Ingredient as provided in the Subject of Report dialog box.
You can choose whether to view these field or not. Check the checkbox displayed with this field to hide or view it. |
| Trade Name | Automatically displays the Trade Name(s) as provided in the Subject of Report dialog box. Multiple trade names are also displayed together, separated by commas.
You can choose whether to view this field or not. Check the checkbox displayed with this field to hide or view it. |
| International Birth Date | Automatically displays the earliest license awarded date, when a user selects an Ingredient and a Product.
You can choose whether to view this field or not. Check the checkbox displayed with this field to hide or view it. |
| Print all configuration criteria on separate cover page | Mark this box to print out the configuration of this report when the report is printed. This is only available when PDF option is selected during printing. |
| Print page numbers on reports | When checked, this option enables the user to print page numbers on a periodic report. This is the default for all report configurations.
If this checkbox is not checked, the following occur.
|
| Allow access to report cases through Hit List | When the report is run as final, it creates a Hit List, which can be retrieved from other areas of the application where advanced conditions can be selected. Click this checkbox to report cases through the Hit List. |
Select Products to include in ICH PSUR
| Field | Description |
|---|---|
| Available Ingredients | Displays the list containing the Ingredients used for the product configuration. Select an ingredient from the list displayed in Available Ingredients and click Add/Remove to add/remove the ingredient.
You can select multiple ingredients at a time. |
| Indication | This list contains the Indication configured for the product containing the ingredients in the Available Ingredients section. The selections made from this list get displayed in the Available Products section.
You can select multiple Indications from the list at a time by pressing the CTRL key and clicking the different Indication entities. |
| Formulation | This list contains the Formulation configured for the product containing the ingredients in the Available Ingredients section. The selections made from this list get displayed in the Selected Products section.
You can select multiple Indications from the list at a time by pressing the CTRL key and clicking the different Indication entities. |
| Available Products | This list is automatically populated with the selections made in the Indication section. |
| Selected Products | This list contains products selected by the user from the Available Products list. When a product is selected, the Trade Name field and International Birth Date fields are auto-populated with the license trade name (formulation, concentration) and earliest License Award Date for the product. |
Select Inclusion Criteria
The Inclusion Criteria tab allows you to select search parameters for inclusion of cases in a periodic report. The top section of the dialog box allows you to specify the type of cases that are to be included in the periodic report.
Click Add to add a criterion. Select appropriate items from the list of items that appear.
| Field | Description |
|---|---|
| Use Assessments in Cases | When selected, the PSUR Report will use the Case Event Assessment when performing datasheet listedness calculations. |
| Re-assess cases against datasheet in effect at the beginning | When selected, the PSUR will re-assess the cases in the line listing based on the Active Datasheet on or before the Start Date of the Reporting period. |
| Re-assess cases against datasheet in effect at end | When selected, the PSUR will re-assess the case in the line listing based on the Active Datasheets on or closest to the end date of the PSUR Reporting end date range without exceeding that date. |
| Expeditable Only | This checkbox is available only when an agency is selected in the Subject of Report tab. If you check this checkbox, only the cases classified as submitted expedited reports to the specified agency are used. |
| Exclude Follow-up Cases | Filters out follow-up Reports from the PSUR Line Listing Report. |
| Include Unlocked Cases | Allows you to include unlocked cases in the periodic report. |
| Advanced Condition | Allows you to specify an advanced condition that must be satisfied by each case that is included in the report. Ensure that the advanced condition or the advanced condition query set that is specified here does not contradict any other criteria specified in the dialog box. |
| Use Datasheet Assessment for UDF Tabulations | Allows you to select datasheet for a report to make UDF tabulations.
If no datasheet is selected, the most conservative listedness is chosen, i.e. Unlisted followed by Listed. |
| Add Cases not included in previous reporting period | Allows you to add cases which were not included in the previous reporting period.
You can enter the start date of the period in the Start Date field. |
| Dropdown list | Select the appropriate report type from the drop-down list. |
| Datasheet | This list allows you to specify which datasheet is to be checked to determine the listedness (listed or unlisted) of the case. |
| Serious/Non-Serious | If you select Serious and clear Non-serious, only cases having a serious event are included and vice-versa. If you select both Serious and Non-Serious the seriousness criteria is ignored. |
| Fatal/Non-Fatal | Select Fatal when at least one event has an event outcome of Fatal. If not, select Non-Fatal. If you select both Fatal and Non-Fatal, both types of cases are included. |
| Listed/Unlisted | Select Listed to view only Listedness values. If you select both Listed and Unlisted, all Listedness values (including Unknown) are included. |
| Related/Non-Related | Relatedness refers to the more conservative of reported or company causality. Select Related for any reportable causality type, and Non-Related for any non-reportable causality type. |
| HCP/Non-HCP | HCP refers to cases that identify a Health Care Professional in the Reporter section within the General tab of the Case Form. |
| Primary Reporter Only | This checkbox displays whether the Primary Reporter has been selected to determine the HCP status. |
Include Line Listing
| Field | Description |
|---|---|
| Include Line Listing | Allows you to select whether you want the Line Listing Data Elements printed with the PSUR Report. |
Add Data Elements
-
Select the checkbox displayed against each data element to add it to the report.
-
The unavailable fields are printed on the report by default and cannot be changed.
View Selected Data Elements
This section lists the selected elements and enables you to arrange the order in which these are to be printed.
| Field | Description |
|---|---|
| MedDRA Hierarchy from Cases/Dictionary | Select Cases to populate the data from the case data. Select Dictionary to populate the data from the MedDRA dictionary. |
| Print Only the Term (Preferred Term or Lower Level Term) | Prints only the event Preferred Term (PT) or event Lower Level Term (LLT) as per the selected radio button. Select the PT option to print only the preferred term and not the verbatim description. |
| Print Dose Text in place of regimen dose | Prints the dosage and frequency information from Dose Description field instead of Regimen Dose. |
| Indicate if case was expedited previously | This checkbox is selected if an agency has been selected in the Subject of Report tab. Cases for which an expedited report was previously submitted to the selected authority are marked with an asterisk and the date of submission appears in the line listing.
Any case that has been previously expedited to a selected agency, is listed in the list of Agencies in the Subject of Reports tab. |
| English Language | Provides the option to print the descriptions in English |
| Local Language | Allows a user to specify which Local language for a multi-language field is to be printed i.e. the Abbreviated Narrative field. |
| Print event info (Serious, Un-listed, Related) as Column | Check this checkbox to print the Seriousness, Listedness and Causality under the Event Verbatim column.
Events having listedness of 'Unknown' are considered 'Unlisted'. If only diagnoses are assessed for event assessment, the events which are associated with a Diagnosis but have been marked with Diagnosis as 'No' display '-' for both listedness and causality. |
| List cases only once, under the primary event | Select this option to view the details of cases in the Main Line Listing only once under the Primary Event |
| List cases under all events, details under the primary event | Select this option to view the details of cases in the Main Line Listing only once under the Primary Event, while non-primary events are listed under their respective event hierarchy with a reference to the primary event body system. Therefore, use this option when grouping on Main Line Listing is by the Event Body System. |
| Print Product Indication for the Product selected in the Report | Enables you to print the product indication for the product selected in the report. |
Group Cases
| Field | Description |
|---|---|
| Available Groupings | Allows a user to group cases together from the given list. Select the desired groupings from the list and click Add to move the grouping to the Selected Groupings list. |
| Selected Groupings | Lists the added groupings, and reports the groups in the order they were selected. Up to 5 grouping options can be selected from the Available Groupings list. |
| Available Sortings | Allows a user to sort cases together from the given list. Select the desired sortings from the list and click Add to move the sorting to the Selected Sortings list. |
| Selected Sortings | Allows a user to further sort cases without a total count for each sorted item. Up to 3 sorting options can be selected from the Available Sortings list. This list is populated with the Mandatory Line Listing entities plus any optional data elements chosen for this configuration. |
Specify Summary Tabulations for Line Listing
The Summary Tabulations tab enables you to specify which summary tabulations/listings will appear along with the line listing.The system enables you to separate the cumulative summary by seriousness, relatedness, and listedness. If you choose this option, the system separates the product event detail into the following categories: Serious/Non-Serious, Related/Non-Related, and Unlisted/Listed events.
| Field | Description |
|---|---|
| Include Index of Cases in PSUR | Create an index page of case numbers, for all cases included in the PSUR. |
| Include Summary of Cases Missing Assessments | This option creates a sub-report of cases missing one of the following items:
Click this checkbox to create one or both of the following sub reports: Cases Missing Assessments - This sub-report displays cases that have been included in the PSUR line listing, but one or more of the following have not been assessed:
Cases Not Included in Report - This sub-report displays cases that have not been included in the PSUR line listing as a result of missing one or more of the following items:
|
| Include Line Listing Tabulation | Check this checkbox to view a pre-defined summary tabulation of Report type, Seriousness and Listedness of all cases in the PSUR. |
| Additional Expedited Report Forms (CIOMS/MedWatch/VAERS) | Allows you to print CIOMS/MedWatch/AERS forms (as per selection) for its respective regulatory agency (as selection), for all cases in the report. It also provides an option to have (or not have) a watermark in the forms. |
Include CIOMS Reports
| Field | Description |
|---|---|
| Print CIOMS reports for serious/unlisted cases | Allows a user to print CIOMS I forms for all Serious/Unlisted (Case Level) cases appearing in the PSUR.
CIOMS contain Internal or Other text printed on them when the PSUR is printed using the Internal or Other option. |
| Include Periodic Numbering on the CIOMS reports | Numbers the requested CIOMS I with a periodic format. (i.e. A-1-1 of 2, A-1-2 of 2, A-2-1 of 1, A-3-1 of 1 etc.). The index is modified to also contain the Periodic paging of each CIOMS report. |
Add PBRER Section 6.2 - Cumulative Summary Tabulations of SAEs from clinical trials
| Field | Description |
|---|---|
| Include Section 6.2 | Check this checkbox to include Section 6.2. By default, this is unchecked. |
| Cumulative Start Date | Enter the cumulative start date in this field. This field users to specify the Start Date of the Cumulative date range. If not specified, the start date is picked from the date specified in the Inclusion Criteria. If specified, the application ensures that the date entered is a valid date, and is before the Start Date specified in the Inclusion Criteria. |
| Identify Study Cases using Report Type | Allows you to select the study cases that use report type from the multi-select list box. The values that are populated here, come from the Report Type codelist where the values display the This type includes cases from clinical trials as checked. |
| Group by Comparator | If the Product Type attribute is present in the Study Configuration for the Product being evaluated, this option is displayed. By default, this is unchecked. If this checkbox is selected, it displays sub columns based on Active Comparator names if Group by Comparator is selected. If Group by Comparator is not selected, the count is displayed under the name of the respective active comparator. |
Add PBRER Section 6.3 - Cumulative and Interval Summary Tabulations from Post-Marketing Data Sources
| Field | Description |
|---|---|
| Include Section 6.3 | Check this checkbox to include Section 6.3. By default, this is unchecked. |
| Cumulative Start Date | Enter the cumulative start date in this field. This field users to specify the Start Date of the Cumulative date range. If not specified, the start date is picked from the date specified in the Inclusion Criteria. If specified, the application ensures that the date entered is a valid date, and is before the Start Date specified in the Inclusion Criteria. |
| Identify Spontaneous Cases using Report Type | Allows you to select the study cases that use report type from the multi-select list box. The values that are populated here, come from the Report Type codelist where the values display the This type includes cases from clinical trials as NOT checked. Only those values are included, where Display is set to Yes, in order to be consistent with the Report. |
| Identify Non-interventional Studies (UNION of rules below) | This is a mandatory field, comprising three new rules to identify Non-Interventional Studies: (Case Classification, Report Type, and Observe Study Type). These three new rules are displayed as checkboxes and have a section label called Identify Non-interventional Studies (UNION of the rules below).
This field is mandatory, which means that at least one of the options must be selected if Include Section 6.3 is selected. Along with the three options, there is a multi-select list box for each that is populated with values from the respective codelist (given below) for the corresponding option that the user selected: a. Case Classification is populated with values from Case Classification codelist. b. Report Type is populated with values from Report Type codelist c. Observe Study Type is populated with values from Case Classification codelist with ICSR code values that are not-null All values are included in this list, irrespective of the Display field for Case Classification and Observe Study Type codelists. Those values where Display for Report Type codelist is set to Yes, are also included. A + sign is displayed as a label, next to these options to indicate that it is a UNION of selections. |
Add Cumulative Summary
| Field | Description |
|---|---|
| Include Cumulative Summary | Select the checkbox to create a sub-report count of events, grouped by Product and Body System (SOC) and sorted by Preferred Term. The sub-report contains a previous date range count of events (comparative date range), a current date range count (current PSUR date range) and a cumulative count (all dates) of events assessed against the product(s) of the PSUR and matching the inclusion criteria. |
| Comparative Date Range | Allows a user to specify the previous date range as a comparison date for the events counted, and therefore should not overlap with the current date range specified on the PSUR inclusion criteria tab. |
Add FDA PSUR Support Information
| Field | Description |
|---|---|
| FDA PSUR Support Section
This section can be used if the company has obtained an FDA waiver to submit a PSUR instead of an NDA report. |
|
| Include Adverse Event Summary | Select this option to generate a sub-report of events from the line listing. This sub report is grouped by Body System and Preferred Term. |
| Causality | Select the desired causality from the list.
Ignore - Counts events regardless of causality assessment. Causal - Counts events where the causality is considered reportable in the Causality Category configuration in List Maintenance. Not Causal - Counts events where the causality is considered non-reportable in the Causality Category configuration in List Maintenance. As Determined - Counts events where 'As Determined' causality meets the above selected causality criteria. As Reported - Counts events where 'As Reported' causality meets the above selected causality criteria. Both - Counts events where both 'As Reported' and 'As Determined' causality meet the above causality criteria. Either - Counts events where either the 'As Reported' or 'As Determined' causality meets the causality criteria. |
| Only Cases with HCP Reporter | Check this checkbox to include events for only those cases that feature an HCP reporter |
| Diagnosis | Select this option to ensure that only events marked as diagnosis are counted. |
| Diagnosis & Symptoms | Select this option to ensure that all events are counted in the sub-report. |
| Separate Diagnosis & Symptoms | Select this option to include diagnosis and symptoms separately in the sub-report. Selecting this option means that the case numbers are separated by Diagnosis and Symptoms respectively. |
| Domestic Consumer Support | Select this option to enable domestic consumer support. |
| Print Unsubmitted | This option allows a user to print MedWatch or VAERS forms for U.S. cases |
| Exclude Reports that are Non-Serious and Listed | Allows a user to suppress MedWatch or VAERS forms from printing for Non-Serious listed cases where all events are non-serious and listed for the datasheet specified. |
| Use Periodic numbering on the Reports | Numbers the requested forms with a periodic format. (i.e. Periodic Page 1 - 1, Periodic Page 1 - 2, Periodic Page 2 - 1, Periodic Page 2 - 2, etc.) An index with the Case Number is also included. |
Generate Single Case Submission Report
| Field | Description |
|---|---|
| Single Case Submission Support Section | |
| Generate Periodic ICSR Submissions for any cases in this Periodic Report that does not have at least one scheduled single-case report during the reporting period to the following Reporting Destination(s): Modify | Check this checkbox to generate the ICSR Reports only for the cases, where a Periodic ICSR Report for the message type chosen, does not exist.
Select one or more trading partners from the list box. Important: Any case that does not have an expedited or single case periodic submission to a trading partner, must have an ICSR report scheduled as a part of the Periodic submission. Click Modify to select a different Reporting Destination. |
| Schedule these single-case Periodic Reports to the following Reporting Destination | Select a single-destination trading partner for Periodic Reports from the drop-down list box. |
| Using the Message Type | Select the required message type from the drop-down list box |
Select Summary Listing
The UD Summaries tab allows you to specify which summary listings will appear along with the line listing.
| Field | Description |
|---|---|
| Include these summary tabulations/listings based on the set of cases presented in the line listing | Allows you to select from pre-configured summary tabulations/listings. These tabulations are based only on the data included in the line listing. Selecting the Exclude Follow-up Cases checkbox filters out follow-up cases from the attached report.
If the Exclude Follow-up Cases option is selected on the Inclusion criteria tab, this option is ignored and follow-up cases are always filtered out. |
| Include these summary tabulations based on all cases | Allows for additional sub-reports based on the 'Case Data Analysis' template, to be included as an output for the all cases in the database that meet the PSUR inclusion criterion for all dates. |
| Include these summary tabulations/listings based on the Date Range | This option allows for additional sub-reports based on Case Data Analysis, Case listing or CIOMS II line listing reports to be included as an output for the cases meeting the PSUR inclusion criterion for the Date Range specified when adding the sub-report.
The dates are based on either the Case Creation Date or the Initial Receipt Date as entered on the PSUR Inclusion Criteria tab. Check the checkbox to the right of the sub-report to ignore considering follow-up cases for the sub-report. |
| Identify SUSAR and Special Interest Events | Enables you to identify any SUSAR and special interest events. |
| Additional Separate Page Numbering for Summaries | Enables you to include additional separate page numbering for summaries. |
Schedule the Report
Configure Security Level of the Report
Prepare Content for a US IND Periodic Report
The system enables you to define an IND summary report. You can add a new report as well as copy, modify and delete existing reports.
Create New IND Report
-
Select Reports > IND Reports to open IND Subject of Report view.
-
Click New Report.
OR
Select an existing report from the list and click Copy or Modify.
-
When you click New Report, the IND Line Listing Reports dialog box opens.
-
Enter an appropriate name for the report under Report Name.
-
Use the tabs in this dialog box to configure the IND Report.
-
From each tab in the IND Summary Report, you can choose to Print all configuration criteria on separate cover pages (PDF Only).
Configure Subject in the Report Header
The Subject of Report tab is used to configure the report header and to specify the agency, products, and other elements. Select multiple ingredients for a configured IND Report to view the multiple licenses to be selected for the report.
| Field | Description |
|---|---|
| Primary Agency | Select the Primary Agency. |
| Available Reporting Destinations | Displays the list of configured Regulatory Agencies. Select an agency from the list displayed in Reporting Destination and click Add to add the report to the Selected Destination list.
Select multiple agencies by holding the CTRL key when you click them. |
| Selected Reporting Destinations | Displays the list of agencies where the report is being sent. Select an agency from the list displayed in Reporting Destination and click Add to add the report to the Selected Destination list.
Select multiple agencies by holding the CTRL key when you click them. Likewise, select an agency from the Selected Destination list and click Remove to prevent it from being sent to the selected destination. |
| Company Name | If a regulatory agency is selected in the Subject of Report tab, then the company name associated with the regulatory agency (this association is created by the Administrator) is automatically entered in this field. |
| Product Name | This field is automatically filled as per the Ingredient field.
Check the checkbox corresponding to this field to choose whether you want this field to appear on the report. |
| Approval | This field is automatically filled with License numbers, separated by commas. This is an editable field. |
| Award Date | This field is populated with the earliest awarded Investigational License for US amongst the licenses selected. This field cannot be edited.
Check the checkbox corresponding to this field to choose whether you want this field to appear on the report. |
| Print all configuration criteria on separate cover page | Click this checkbox to print out the configuration of this report when the report is printed. This is only available when the PDF option is selected during printing. |
| Print page numbers on reports | When checked, this option enables the user to print page numbers on a periodic report. This is the default for all report configurations.
If this checkbox is not checked, the following occur.
|
| Allow access to report cases through Hit List | When the report is run as final, it creates a Hit List, which can be retrieved from other areas of the application where advanced conditions can be selected. Click this checkbox to report cases through the Hit List. |
Select Products to Generate the Report
Use the Product Selection tab to select product information to include in the report.
| Field | Description |
|---|---|
| Available Ingredients | Displays the list containing the Ingredients used for the product configuration. Select an ingredient from the list displayed in Available Ingredients and click Add/Remove to add/remove the ingredient.
You can select multiple ingredients at a time. |
| Filter | Enter an Ingredient name and click Filter to search for the entered ingredient within the Available list of Ingredients. |
| Selected Ingredients | Displays the list of ingredients selected from the Available Ingredients list. |
| Indication | This list contains a list of all the indications for the products containing the selected ingredient. The selections made from this list are displayed in the Available Products section.
You can select multiple Indications from the list at a time by pressing the CTRL key and clicking the different Indication entities. |
| Formulation | This list contains the Formulations configured for the product containing the selected ingredient and indication. The selections made from this list get displayed in the Selected Products section.
You can select multiple Formulations from the list at a time by pressing the CTRL key and clicking the different Indication entities. |
| Available Licenses | This list is automatically populated with the licenses from the Indication section. |
| Selected Licenses | This list contains licenses selected by the user from the Available Licenses list. When a product is selected, the Trade Name and International Birth Date fields are auto-populated with the license trade name (formulation, concentration) and earliest License Award Date for the product. |
Select Inclusion Criteria
The Inclusion Criteria tab allows you to select search parameters for inclusion of cases in a periodic report.
| Field | Description |
|---|---|
| Case Creation Date | Allows you to specify a range of cases by the date when the case was created. |
| Case Receipt Date | Allows you to specify a range of cases by the initial receipt date. |
| Use Current Version | Allows you to use the latest revision to populate the data within the selected reports. |
| Use DLP Version | Allows you to use the case data of the version as of the specified DLP Version. |
| Age Groups | Allows you to include or exclude cases based on the patient's age group. Select the Age Groups checkbox to activate the age groups and select all the age group categories that apply. |
| Options - Domestic/Foreign Cases | This option allows the user to include domestic and foreign cases within the periodic report. Select Domestic if Country of Incidence is USA and Foreign if Country of Incidence is not USA. |
| Expeditable Only | This checkbox is available only when an agency is selected in the Subject of Report tab. If you check this checkbox, only the cases classified as submitted expedited reports to the primary agency are used. |
| Include Unlocked Cases | Allows you to include unlocked cases in the periodic report. |
| Advanced Condition | Allows you to specify an advanced condition that must be satisfied by each case that is included in the report. Ensure that the advanced condition or the advanced condition query set that is specified here does not contradict any other criteria specified in the dialog box. |
Include Line Listing
| Field | Description |
|---|---|
| Report All Events | Select this option to report all events. |
| Report only Diagnosis Events | Select this option to report only diagnosis events (only the diagnosis events that are either explicitly marked as diagnosis or are non-related symptoms). |
| List cases only once, under the primary event | Select this option to view the details of cases in the Main Line Listing only once under the Primary Event |
| List cases under all events, details under the primary event | Select this option to view the details of cases in the Main Line Listing only once under the Primary Event, while non-primary events are listed under their respective event hierarchy with a reference to the primary event body system. Therefore, use this option when grouping on Main Line Listing is by the Event Body System. |
| Create a Sub Report for Death Cases | Check this checkbox to separate death cases from the main IND listing. If the checkbox is checked, all death cases (Identified by any event marked as death in Seriousness Criteria or any event having a Event Outcome as Death) are filtered out from the IND Line Listing. All death case show up in a sub report, called IND Line Listing (Death Cases). |
Specify Summary Tabulations for Line Listing
The Summary Tabulations tab enables you to specify which summary tabulations will appear along with the line listing.
| Field | Description |
|---|---|
| Include these summary tabulations/listings based on the set of cases presented in the line listing | Allows you to select from pre-configured summary tabulations/listings. These tabulations are based only on the data included in the line listing. Select the Exclude Follow-up Cases checkbox to filter out follow-up cases from the attached report.
If the Exclude Follow-up Cases option is selected on the Inclusion criteria tab, this option is ignored and follow-up cases are always filtered out. |
| Include these summary tabulations based on all cases | Allows for additional sub-reports based on the 'Case Data Analysis' template, to be included as an output for the all cases in the database that meet the IND Report inclusion criterion for all dates. |
| Add | Displays a list of memorized Case Data Analysis Reports that have been marked for availability in a periodic report. |
| Remove | Click this button to remove a selected report. |
| Additional Separate Page Numbering for Summaries | Enables you to include additional separate page numbering for summaries. |
| Include Summary of Unlocked Cases | Enables you to print the list of Case Numbers that are included in the Periodic Reports but are not locked. |
Prepare Content for an NDA Periodic Report
The NDA Periodic Reports enable you to define an NDA Periodic report. You can add a new report as well as copy, modify and delete existing reports.
-
You can print an Index of Cases included in the NDA report.
-
If you select this option, the system lists the cases from the following sections once at the end of the configuration pages:
-
Sequential List of cases
-
Serious Listed Initial/Follow up
-
Non Serious Listed Initial/Follow up
-
Non Serious Unlisted Initial/Follow up
-
15 Day Submission
-
-
The page numbering for this sub-report continues from the configuration pages.
-
You can separate initial case events from follow-up case events in the Summary Tabulation tab of the NDA Report.
-
If you select this option, the system counts events in the Initial section if the case is in the Serious Listed, Non-Serious Listed, or Non-Serious Listed/Unlisted sections.
-
If you select this option, the system counts events in the Follow-up section if the case is in the Serious Listed or Non-Serious Listed/Unlisted follow-up sections of the NDA report.
-
For the 15 Day events, if the case has not been previously reported in a NDA, the system counts it in the Initial section then the Follow-up section.
-
If you select List cases once under the Primary Event System Organ Class (SOC), the system displays a footnote with an asterisk ( * ) printed across all the System Organ Classes on the report and the following statement: Primary Event System Organ Class.
-
-
If you select the Print FDA-3500A/VAERS form at the end option, the system prints the report sections in the following order:
-
Configuration (Including Case Indices (e.g. Sequential Case Listing, Listing by Seriousness/Listedness, Listing of Cases Missing Analysis)
-
Line Listing
-
Summary Tabulations
-
MedWatch/VAERS reports at the end of the report
-
-
Page numbering for the MedWatch reports continue from the last page of the NDA report.
-
The configuration pages have been updated to reflect the updates made to the NDA Reports
-
The configuration pages are printed at the beginning of the NDA report.
-
By default, these are unchecked on all the existing configured reports.
Create NDA Summary Report
-
Select Reports > Periodic Reports > NDA Reports to open the NDA Subject of Report view.
-
Click New Report to create an entirely new report,
OR
Select an existing report from the list and click Copy or Modify.
-
When you click New Report, the NDA Line Listing Reports dialog box opens.
-
Enter an appropriate report name in the Report Name field
-
Use the tabs in this dialog box to configure the NDA Report.
-
From each tab in the NDA Report, you can choose to Print all configuration criteria on separate cover pages (PDF Only).
Configure Subject in the Report Header
On the Subject of Report tab you can select multiple Ingredients for a configured NDA Report per allowable variations of product and license configuration and periodic reporting requirements for the FDA. Select multiple ingredients to view the multiple licenses to be selected for the report.
| Field | Description |
|---|---|
| Available Reporting Destinations | Displays the list of configured Regulatory Agencies. Select an agency from the list displayed in Reporting Destination and click Add to add the report to the Selected Destination list.
Select multiple agencies by holding the CTRL key when you click them. |
| Selected Reporting Destinations | Displays the list of agencies where the report is being sent. Select an agency from the list displayed in Reporting Destination and click Add to add the report to the Selected Destination list.
Select multiple agencies by holding the CTRL key when you click them. Likewise, select an agency from the Selected Destination list and click Remove to prevent it from being sent to the selected destination. |
| Company Name | If a regulatory agency is selected, the company name associated with the regulatory agency (this association is created by the Administrator) is automatically entered in this field. |
| Ingredient | This field is populated with ingredient selected in the Subject of Report tab.
Check the checkbox corresponding to this field to choose whether you want this field to appear on the report. |
| Approval | This field is automatically filled with License numbers, separated by commas. This is an editable field. |
| Trade Name | Automatically displays the Trade Name.
Multiple trade names are also populated from license trade name (formulation, concentration) of selected licenses, separated by commas. Check the checkbox corresponding to this field to choose whether you want this field to appear on the report. |
| Award Date | Automatically displays the earliest license awarded date, when a user selects an Ingredient and a Product.
Check the checkbox corresponding to this field to choose whether you want this field to appear on the report. |
| Print all configuration criteria on separate cover page | Click this checkbox to print out the configuration of this report when the report is printed. This is only available when the PDF option is selected during printing. |
| Print page numbers on reports | When checked, this option enables the user to print page numbers on a periodic report. This is the default for all report configurations.
If this checkbox is not checked, the following occur.
|
| Allow access to report cases through Hit List | When the report is run as final, it creates a Hit List, which can be retrieved from other areas of the application where advanced conditions can be selected. Click this checkbox to report cases through the Hit List. |
Select Products to Generate the Report
The Product Selection tab enables you to select product information to include on the report.
| Field | Description |
|---|---|
| Available Ingredients | Displays the list containing the Ingredients used for the product configuration. Select an ingredient from the list displayed in Available Ingredients and click Add/Remove to add/remove the ingredient.
You can select multiple ingredients at a time. |
| Filter | Enter an Ingredient name and click Filter to search for the entered ingredient within the Available list of Ingredients. |
| Selected Ingredients | Displays the list of ingredients selected from the Available Ingredients list. |
| Indication | This list contains a list of all the indications for the products containing the selected ingredient. The selections made from this list are displayed in the Available Products section.
You can select multiple Indications from the list at a time by pressing the CTRL key and clicking the different Indication entities. |
| Formulation | This list contains the Formulations configured for the product containing the selected ingredient and indication. The selections made from this list get displayed in the Selected Products section.
You can select multiple Formulations from the list at a time by pressing the CTRL key and clicking the different Indication entities. |
| Available Licenses | This list is automatically populated with the licenses from the Indication section. |
| Selected Licenses | This list contains licenses selected by the user from the Available Licenses list. When a product is selected, the Trade Name and Award Date fields are auto-populated with the license trade name (formulation, concentration) and earliest License Award Date for the product. |
Select Inclusion Criteria
| Field | Description |
|---|---|
| Case Creation Date | Allows you to specify a range of cases by the date when the case was created. |
| Case Receipt Date | Allows you to specify a range of cases by the initial receipt date. |
| Use Current Version | Allows you to use the latest revision to populate the data within the selected reports. |
| Use DLP Version | Allows you to use the case data of the version as of the specified DLP Version. |
| Age Groups | Allows you to include or exclude cases based on the patient's age group. Select the Age Groups checkbox to activate the age groups and select all the age group categories that apply. |
| Option (Applicable to Non-15-Day Selection Only)- Domestic/Foreign Cases | This option allows the user to include domestic and foreign cases within the periodic report. Select Domestic if Country of Incidence is USA and Foreign if Country of Incidence is not USA. |
| Option (Applicable to Non-15-Day Selection Only)-
Exclude Literature Cases/Study Cases |
This option allows the user to exclude Literature and Study Cases from being considered for the NDA Report. Select Exclude Literature Cases to exclude literature cases and select Exclude Study Cases to exclude study cases. |
| Advanced Condition | Allows you to specify an advanced condition that must be satisfied by each case that is included in the report. Ensure that the advanced condition or the advanced condition query set that is specified here does not contradict any other criteria specified in the dialog box. |
| Add Cases not Included in a previous reporting period Start Date | Enter the start date. This adds cases not included in a previous reporting period with the specified start date. |
| Include Unlocked Cases | Allows you to include unlocked cases in the periodic report. |
| Evaluate Primary Suspect Drug Only | Allows you to select only the Primary Suspect Drug. |
Include Line Listing
The NDA report comprises of three tabs. The options for these tabs can be configured in the Line Listing tab.
| Field | Description |
|---|---|
| Tab 1: FDA - 3500/VAERS Forms | Check this checkbox to generate the MedWatch 3500A (Drug) or VAERS reports which are serious listed or non-serious |
| Suppress printing of non-serious listed reports | Check this checkbox to prevent printing the non-serious listed reports but print their case numbers in the main NDA report indices
Tab 1 of the NDA Line Listing report cannot be generated without Tab 2. However, Tab 2 can be generated without Tab 1 |
| Tab 2: Index of Submitted Forms in Tab 1 | Check this checkbox to generate an index of the forms from Tab 1 It prints all MedWatch/VAERS forms for the following cases:
Previously expedited 15-day reports that are Serious and Unlisted that have already been submitted to the FDA do not need to be re-submitted with this periodic report |
| Tab 3 Part 1: NDA Line Listing of 15 Day Reports Submitted | Check this checkbox to generate a list of all serious unlisted expedited reports within the specified time period.
The dates in these reports are in GMT. |
| TAB 3 Part 2: Tabulation by System Organ Class (SOC) of All Event Reports Submitted | Check this checkbox to generate a tabulation by System Organ Class (SOC) of all events reported during the specified time period. This includes the cases for which expedited reports were previously generated, as well as the cases that are submitted as part of the current report |
| TAB 3 Part 3: Cases sent to FDA under another NDA | Check this checkbox to print a list of all the serious unlisted events for which reports were submitted to the FDA previously
Note: If you select to print out the Tab 3 Part 3 section, the NDA report looks for other submissions (E2B, MW, MW Drug, VAERS, eVAERS, eMDR) to the same agency for the same case against other (not included in selection criteria for this report) marketed licenses. Any submission matching this criterion is listed on the Tab 3 Part 3 section of the NDA report. If there are multiple submissions against different licenses, then each one is listed. Each license is listed only once |
| Include Periodic Submissions | Check this checkbox to include all cases that have been sent under another NDA |
| Start Page Number | Select the page number for the first page of the report |
| Listing Options | These options for List cases only once, under the primary event body system and List cases under all events body systems only apply to the NDA Line Listing of Expedited Reports Submitted report |
| List cases only once, under the primary event System Organ Class | Select this option to list cases only once |
| List cases under all events System Organ Classes | Select this option to list cases under each SOC for each event |
| Include Summary of Cases Missing Assessments | Check this checkbox to include a Summary of Cases missing Assessments at the end of the report |
| Include Summary of Unlocked Cases | Select this option to include a summary of unlocked cases |
| Include Listing of Nullified 15-day Alert Cases Submitted During the Reporting Period | Any case that was deleted which has submitted report within the reporting period after an expedited submission was made to the FDA (primary agency) for the license specified in the NDA Periodic report, regardless of the report form used, e.g., it could be MedWatch or Expedited E2B (E2Bs with MESSAGETYPE tag value configured as Expedited in Message Type Code List) or eVAERS. |
| Use Periodic numbering on the Reports | Select this option to use periodic numbering on reports |
| Custom Case Summary Tabulation | Enter the Summary Report Title |
| Advanced Condition | Select the Advanced Condition from the drop-down list |
Specify Summary Tabulations for Line Listing
The Summary Tabulations tab allows you to specify which summary tabulations will appear along with the line listing.
| Field | Description |
|---|---|
| Include these summary tabulations/listings based on the set of cases presented in the line listing | Allows you to select from pre-configured summary tabulations/listings. These tabulations are based only on the data included in the line listing. Select the Exclude Follow-up Cases checkbox to filter out follow-up cases from the attached report.
Note: If the Exclude Follow-up Cases option is selected on the Inclusion criteria tab, this option is ignored and follow-up cases are always filtered out. |
| Include these summary tabulations based on all cases | Allows for additional sub-reports based on the 'Case Data Analysis' template, to be included as an output for the all cases in the database that meet the NDA Report inclusion criterion for all dates. |
| Add | Displays a list of memorized Case Data Analysis Reports that have been marked for availability in a periodic report. |
| Remove | Click this button to remove a selected report. |
| Additional Separate Page Numbering for Summaries | Enables you to include additional separate page numbering for summaries. |
| Case Count Summary Report | Enables you to print the list of Case Numbers that are included in the Periodic Reports but are not locked. |
View Scheduled Periodic Report Information
Available User Options
Common features on the Period Reports page. Click the icon associated with each report to view the following options:
| Option | Description |
|---|---|
| Description | Displays the report name. Click this to open the selected report in PDF format. |
| Status | Opens the Report Details dialog box for the selected report. |
| Print List | Allows the user to print the current Periodic Reporting for referencing the current view of the Periodic Reporting. |
| View Report | Opens the Individual Periodic Report selected by the user. |
| Report Details | Displays specific information about the report as entered in the Regulatory Reports section.
The information displayed in the fields of the Report Details dialog box is fetched from the data entered in the Regulatory Reports section of Case Form. |
View Report Details
The Report Details > General tab displays the general information about the report. The information on this tab cannot be modified.
| Field | Description |
|---|---|
| Agency | Displays the Reporting Destination for which the report is scheduled. |
| Responsibility | Displays the User Group to which the report is assigned. |
| Case Nullification Date | Displays the date when the case was nullified. |
| Case Nullification Reason | Displays the reason entered when a case is logically deleted in Argus. |
View Report Schedule Details
The Scheduling tab displays a reason for scheduling this report. It also shows the date on which the report was scheduled.
All fields in this tab are auto-populated as per records entered in Argus.
View Report Routing History
The Routing tab displays the routing history of the report. To route the report, click Route.
| Field | Description |
|---|---|
| Group | Displays the group of the report. This button is enabled when you click the Route button. |
| User | Displays the state of the report. This button is enabled when you click the Route button. |
| Comments | Displays routing comments entered before routing the report. |
Submit a Periodic Report
Specify Submission Details
The Submission tab enables you to specify whether submission is required and enter a reason for not submitting the report.
| Field | Description |
| Submission Required | Enables you to select if this report is not required to be submitted to the regulatory authority. |
| Reason for Non-Submission | Click Select to select the reason for non-submission. |
Add Comments
The Comment tab enables you to enter a local comment that prints out on that specific report when generated. Each report has its own Local Comment section.
Transmit a Report
-
Click the icon associated with a report and select the Transmission tab from Report Details.
-
When the system opens the Report Details The Report Details dialog box opens.
-
Click OK or Cancel to approve the transmission or discard any changes, respectively.
-
Click the Transmit button to transmit a report. The Transmit to Recipients dialog box is displayed.
-
Select the recipients of the report, as applicable from the Available Recipients list.
-
Select the method of transmission from Method, as applicable.
-
Enter remarks in Comments.
-
Click Transmit.
-
The selected report is transmitted to the specified recipients.
View Report Generation Status
The Report Generation Status tab is displayed for users who have been configured by the Argus Safety administrator in Argus Console, under Access Management > Groups > Menu > Reports > Periodic Reports and for users who have access to the System Library.
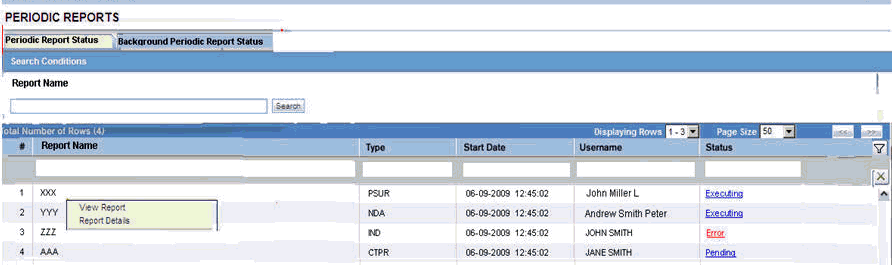
| Field | Description |
|---|---|
| Run At Date | Displays the date and time when the report generation started.
For English users, it is displayed in DD-MMM-YYYY HH:MM:SS (GMT offset) format. For Japanese users, it is displayed in YYYY/MM/DD 00:00 (GMT offset) format |
| Status | Displays the current status of report generation in the form of hyperlinks, with the options listed below:
On clicking the Generated status, the Periodic Report is displayed. On selecting any status other than Generated, the Background Periodic Report Execution Status dialog box is displayed, as shown below: 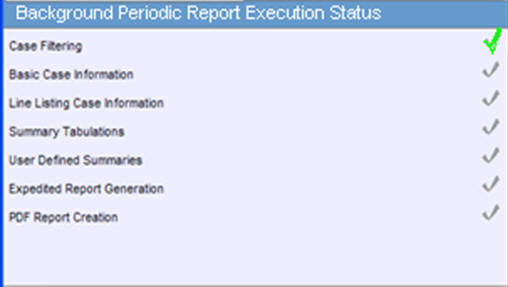
This dialog box displays the different stages of Periodic Report status. A grey tick mark is denotes Pending state, a Green tick mark denotes Completed State, and a Red cross mark denotes an Error. |
| Context Menu > View Report | This option is displayed in the context menu only when PDF/CSV/RTF Report Creation is Generated. On clicking View Report, the selected report is displayed. Applicable only for Periodic reports. |
The Report Status screen displays the report outputs for the logged in user with the last report run as the first report. The reports can be filtered using:
-
Report Name
-
Draft/Final
-
Report Type
-
Scheduled By
-
Scheduled On
-
Run At Date
-
Status
Create Unscheduled Periodic Reports
-
Click Reports > Compliance > Periodic > Create Unscheduled Report.
-
The system opens Periodic Reports dialog box that provides a list of configured reports of the following types:
-
PSUR - Containing ICH PSUR Line Listing Reports
-
IND - Containing US IND Periodic Reports
-
NDA - Containing US NDA Periodic Reports
-
CTPR - Containing CT Periodic Reports
-
-
Click the (+) icon against the desired category to view all the reports within that category.
-
Select the report you wish to create from this list and click Select.
-
When the system opens the Report Batch Printing dialog box, select Run Now or Run at, as appropriate.
Note:
If you select Run Now, specify PDF, RTF, or CSV from the drop-down list for the report output option to generate the PSUR or CTPR report in the selected format.If you select Run at, specify the date/time to schedule the PSUR report to be generated by Argus Safety Service. This enables only Final and disables all other Print As options.
-
In the Email field, enter the email ID of the user to whom the periodic report should be emailed, once completed. By default, this field lists the email address (if configured) of the current user.
-
Select what you want printed on the report: Final, Draft, Internal, or enter Other information.
-
Run Using allows users to select between Argus Native Periodic report and BI Publisher.
-
Report Form Type allows users to select the Report Templates and these options are dependent on the value selected in Run Using. If Run Using is selected as Argus Native, the Report Form Type drop-down list displays PBRER and PSUR. The option selected from this drop-down list is used as Report Form Type for follow-up algorithm.
-
The options displayed in the Report Form Type are configurable through the flexible codelist Report Template.
-
Click OK.
-
The system generates the periodic report.
Approve and Submit a Report
Before you can mark a report as submitted, the report must first be approved.
-
Open the case for which the report has to be approved.
-
Open the Regulatory Reports tab in the Case Form.
-
When the system opens the Regulatory Reports details for the selected case, click the icon associated with the report you wish to approve.
-
Select View Report Details.
Note:
The Case Nullification Date is the date when the case is deleted, the Case Nullification Reason is the comment entered when the case is logically deleted in Argus. -
Select Submitted from the State list in the Routing tab and click Route.
-
When the system opens a dialog box, enter the required details and click OK.
-
The report is approved.
Note:
A user who has Workflow Manager rights can undo the submission of a report, if necessary.View Submitted Reports
-
Select the Reports > Compliance > Submitted Reports.
-
When the system opens the Submitted Reports page, enter the appropriate search criteria and click Search.
-
The system displays the Search Results.
Submitted Reports—Fields and Field Descriptions
| Field | Description |
|---|---|
| Case ID | Enter the specific case number.
Tip: Use wild cards such as 2007% to search for cases starting with 2007. |
| Include these Reports | Select the required report type or case status to be displayed. |
| Product | Select the product as required. The reports scheduled for these products will be displayed. |
Use Bulk Report
Bulk Reporting enables you to print, transmit or submit reports in bulk.
-
Select Reports > Bulk Reporting to view the Bulk Report screen.
-
The system displays the screen.
Filter Bulk Reports
The Bulk Reporting Filter sections enables you to filter reports.
| Field | Description |
|---|---|
| Destination |
|
| Report Status | From the drop-down list, select one of the options:
Note that to display Generated Only or both Scheduled and Generated reports, configure the common profile switch "Allows Bulk Reporting screen to show Generated Reports Only" as follows:
|
| Report Status | Choose either Scheduled/Generated, Pending, Failed, or Printed/Transmitted from the drop-down list. |
| Print Regulatory Report | Prints the report as Draft or Final. The Draft option is disabled when the printing option is set to Transmit.
Select Medical Summary to view the list of only medical summaries of distinct cases in a PDF. |
| Approved Reports Only | Filters reports for only approved reports. |
| View All | Displays the bulk reports applicable to your filter selections. |
| Product Family | Enter a Product family to view all cases where the scheduled reports belong to the searched Product family. |
| Study ID | Filters reports on the basis of the Study ID. |
| Product Group | Filters reports on the basis of the Product Group. If a value is selected in the Product Group filter, the Product Family drop-down list's values automatically narrow down to the product families which belong to the selected Product Group filter. |
| Specific Case # | To search a case, enter the Case Number, and click Retrieve. |
View Bulk Report Filter Results
The filter search results appears in the Total Number of Rows section.
| Field | Description |
|---|---|
| Suspect Product | Displays the Trade Name for which the report has been scheduled. If more than one Suspect Company Product exists for the case, an (+) is placed at the end of the product name.
For Reports which were scheduled for the Device, the Device name is displayed. |
| Diagnosis | Displays the Primary Event Diagnoses PT |
| (Event Verbatim) | Displays the (Verbatim as reported) of the Primary Event. |
| S/U/R |
|
| F or LT | Fatal / Life Threatening
|
| 7/15 | Number of days for which the report is due.
|
| Blind Study Product | Transmits and prints study cases with blinded information. |
| Mark as Submitted |
|
| Report Form | 1. Displays the Description of the report
2. Click the Report form link to view the DRAFT Report as a PDF. |
| Destination | Displays the report destination (agency) for which the report is scheduled. |
| Downgrade | Allows the user to view if the report is downgrade.
Displays Yes if the report is a downgrade report else. |
| View All | Allows administrator and workflow manager to see all items in the system. |
Tip:
The icon (displayed in the lock state) in the Reports > Bulk Reporting screen denotes a SUSAR (Suspected Unexpected Serious Adverse Reaction) case.Print Reports in Bulk
| Field | Description |
|---|---|
| Blind Study Product | Check this checkbox to print study cases with blinded information. |
| Mark as Submitted | Check this checkbox to mark reports as Submitted when the transmission/email has been sent.
A dialog box is displayed is this checkbox is not selected. This dialog box prompts you to confirm if the report is to marked as submitted or not. Select Yes or No, as required. This selection is remembered for the next time when you print a report. |
| Print Medical Summary | Allows the user to print the Medical Summaries. |
| Allows you to choose the printer for the selected report from the Select Site Printer dialog box.
Select the Site and Printer Name where you wish to print the report and click OK. |
|
| Print List | Allows the user to print the current view of the Bulk Reporting. |
Sort Cases
To sort the cases based on the case status, click the Lock State header row.
-
Lock State
-
SUSAR
-
Exp/Per
These options enable you to sort cases based on the case categorization.
Tip:
The icon (displayed in the lock state) in the Reports > Bulk Reporting screen denotes a SUSAR (Suspected Unexpected Serious Adverse Reaction) case.Lock State Icon Options
Click the Lock State icon to view the list of available options.
| Field | Description |
|---|---|
| View Report | Displays the Draft report. |
| Report Details | Displays specific information about the report as entered in the Regulatory Reports section. |
| Case Summary | Displays the Case Summary dialog box. |
| Remove Report | Deletes the report from the case on being asked for a justification. |
| Mark for Non-Submission | Displays the Submission tab in the Report Details dialog box.
Select No for Mark for Non-Submission and enter the reason for the non-submission. |
Track your Periodic Report Submission
Use Submitted Reports Search Results
| Field | Description |
|---|---|
| Time Frame (I/F-u) | Displays the whether the report was initial or follow-up. |
| Product | Displays the first suspect product for the case on which the report is based (expedited reports). |
| License Type | Displays the license type of the report. |
| Primary Event | Displays the first event term for the case on which the report is based (expedited reports). |
| Reason for Scheduling | Displays the reason provided for scheduling the report. |
| Report Form | Displays the type of report scheduled (form) and initial/follow-up status (e.g. "Initial Report" or "Follow-up #3"). |
| Submitted Date | Displays the report's submission date. |
| Case Del. Date | Displays the date when the case was deleted. |
| Blind Study Product | Enables you to mark the study product as blinded. When you manually schedule a report, the system enables you to check Blind Product Study on the Schedule New Expedited report dialog box to blind the study products if they are in the case. |
| Print All Submitted Reports | Enables you to print all the submitted reports. |
Reopen Submitted Reports
Cases that are archived while unsubmitting reports can be reopened from the Archived Case dialog box.
-
Enter the password and notes required in the Archived Case dialog box.
-
When the system opens the Report Unsubmit dialog box, enter the reason for unsubmitting the report and click OK.
-
The system unsubmits the report.
Tip:
The icon (displayed in the lock state column) in the Reports-> Compliance - Expedited and Submitted screens denotes a SUSAR case.