2 Enter case data
Create a new case
When your company receives new adverse event data, you need to enter this information and check for duplicates before saving a case in Argus Safety.
You can create a new case by doing either of the following:
-
Select Case Actions, and click New.
-
Select Worklist, and click Intake.
Enter a new case
From the Initial Case Entry form, you can decide to book in a case before entering further case information in the Case Form.
To enter a new case:
-
On the Home page, hover over the Case Actions menu and click New.
-
On the Initial Case Entry page, enter the mandatory information (fields with a red flag) and click Continue.
-
Click Search to confirm this is not a duplicate case.
-
A list of cases that match the criteria you entered on the form is displayed. Review the search results for a duplicate case.
If a duplicate case exists, open the case to enter further information related to the existing case. Click the link associated with the case.
-
If a duplicate case does not appear, click Continue in the Initial Case Entry form to display the BookIn and Attachments and References sections.
When a duplicate search is performed, the system remembers the results until the user logs out of the system or performs a duplicate search again. When the Bookin screen is opened, the search results from the last search are displayed again.
-
Enter the relevant information in the following sections:
-
Under Reported Causality, select the reporter's assessment of causality. The causality relationship is the causal relationship between the clinically most important event and the suspect drug that is entered in the Initial Case Entry form.
-
For Seriousness Criteria, check the appropriate checkboxes to indicate the seriousness of the case, as appropriate.
-
Attachments and References
-
To insert a file attachment
1. Click Add on the Attachments and References tab.
2. Enter the Classification and Description, as applicable. Select File Attachment from the drop-down list and click Add.
3. On the File Attachment -Web page dialog box, double-click Browse and navigate to the relevant file. Click B.
Contact your system administrator to configure this location.
-
To insert a URL Reference
1. Select URL Reference from the drop-down list.
2. Enter the URL after http://.
-
To search for and insert a document
1. Select Documentum Link and click Add.
2. The Document Lookup dialog box is displayed.
You can search for a document from the database by specifying a search criterion in this dialog box.
-
-
-
Click BookIn.
Once clicked, this button is hidden from the screen. The button is enabled only if the Save operation fails or when the case is booked-in successfully.
-
If Argus has been configured to generate a case number automatically, it is generated now.
If the case number is not automatically generated, enter it according to your company guidelines.
-
When the case creation message appears, click Yes.
Note: If you click Yes, the Case Form page appears. You can now enter case data.
If you click No, the case is saved and closed. You can open the case and enter data at any time.
Initial case entry—fields descriptions
| Field | Description |
|---|---|
| Initial Receipt Date | Enter the complete date on which your company became aware of the case. The Receipt Date cannot be entered as a partial date. |
| Central Receipt Date | Enter the date on which this information was received by Central Safety. |
| Country of Incidence | Select the country where the adverse event occurred. This may or may not be the reporter's or the patient's country of residence. |
| Report Type | Select the item that best describes the type of report. This determines the fields that are made available for entering case information.
The report type also impacts duplicate search. For example, selecting Sponsored Trial makes the Study ID and Protocol ID fields available. The Administrator can adjust the information in this list. |
| Study ID(applicable to clinical trial cases only) | Click Select to choose the study ID from the Clinical Trial Selection dialog box. |
| Center ID
(applicable to clinical trial cases only) |
Click Select to choose the Center ID from the Clinical Trial Selection dialog box. |
| Initial Justification | The values of this field are configurable through the standard Argus Justifications dialog box. This field is reflected in the General tab of the Case Form and is also available for duplicate searching for cases.
Click the green dot to view and select justifications from the Argus Justification dialog box. |
| Product Name | If the adverse event(s) are associated with more than one product, each additional product can be added from the Case Form.
Enter the most suspect product here. Click Select to search for a product from the Trade Name Product Lookup dialog box. Several items are automatically entered on the Case Form based on the product selected here. |
| Generic Name | This field can be used to enter the generic name of the product. This field is automatically entered when a product is selected from the Trade Name Product Lookup dialog box. |
| Description as Reported | Enter a brief verbatim description from the reporter describing the event that is most clinically important in the case. The icon denotes that the event is encoded. Click the icon to populate the MedDRA hierarchy dialog box. |
| Onset Date/Time | Enter the date/time for the onset of adverse event symptoms. |
| ID | Enter the value to search for a Reporter Reference number, Case Reference, and Case number. |
| Journal and/or Title | These items are applicable to literature cases only. Click Select to choose a journal and/or title from the Literature Reference dialog box. The Administrator can adjust this list. |
Check for duplicates
-
Enter the information related to the case in the Initial Case Entry form.
-
Select the Receipt Range Limits option to check for duplicates based on the dates entered in the form and click Search.
-
Review the list to determine if any case contains duplicate information.
Receipt range—fields descriptions
| Field | Description |
|---|---|
| No date | 90 days before System Date and 2 days after System Date. |
| Full Onset Date | 10 days before Onset date and 90 days after Onset Date. |
| Full Initial Receipt Date | 60 days before Initial Receipt Date and 60 days after Initial Receipt Date.
Note: This default date range for searching on Initial Receipt Date can be disabled by un-checking the Receipt Range Limits option on the Initial Case Entry dialog box. |
| Partial Onset Date | Based on the Full Date Range - if only the year is entered, the date range becomes: 10 days before the end of the previous year and 90 days after the end of the year. |
| Partial Initial Receipt Date | Based on Full Date Range - if only a year is entered, the date range becomes: 60 days before the end of the previous year and 60 days after the end of the year. |
Note:
-
If an Initial Receipt Date is not entered but an Event Onset Date has been entered, the search will default to look for cases with Initial Receipt Dates 10 days before and 90 days after the Event Onset Date. This feature can be disabled directly on the dialog box by un-checking the Receipt Range Limits checkbox. If you do not check anything, the default date range is 90 days before and 2 days after the current date.
-
The Duplicate Search permits you to sort in ascending or descending order in the duplicate search results. You can also use wildcard searches on all text fields in the Book-in dialog box.
Initial case entry—BookIn dialog box
-
You can enter the attachment classifications and their descriptions on the Initial Case entry dialog box.
-
The values entered in the Classifications and Description fields are transferred after the cases have been booked.
-
When you book in a clinical trial case and select a study where the country of incidence value does not match the list of countries defined in the study configuration, a warning message appears.
-
Right-click the row, and select one of the following:
-
View Case Summary
-
Print Medical Summary—Displays the Medical Summary report PDF. If you do not have access to the Medical Review dialog box, the Medical Summary Report is hidden.
-
Print—Launches the Case Form print dialog box. If you do not have access to the Case Form print dialog box, the Print Case option is hidden.
-
Accept incoming cases
The Intake function enables you to view a list of incoming attachments in the Worklist View where you can select an attachment for creating a case.
To access the Worklist > Intake page, hover over the Worklist menu and select Intake.
View pending case
Use Pending tab to create a case, reject a case, and so on. Users other than Workflow Managers can reject a case from the Intake Worklist.
| Field | Description |
|---|---|
| Priority | Allows the user to view the priority of the case. |
| Case Type | Allows the user to view report type information. |
| Reporter Type | Allows the user to view the Reporter type for the Primary Reporter in the case. |
| Group | Allows for the current group owner or Unassigned group to be assigned to the case. |
| Central Site/LAM Site | Allows the user to view the current Site (Argus or Affiliate) of the Case - If there are no Sites defined, ALL users can access the case attachment. |
| Attachment Name | Allows the user to view the attachment which is associated to the case. |
| Classification | Allows the user to view the attachment classifications which is associated with the case. |
| Description | Allows the user to view the attachment description which is associated with the case. |
View rejected cases
| Field | Description |
|---|---|
| Generic Name | Allows the user to view the generic name of the suspect product in question. |
| F, LT or H | Allows the user to view Fatal, Life-Threatening or Hospitalized cases. |
| Reporter Type | Allows the user to view the Reporter type for the Primary Reporter in the case. |
| Country | Allows the user to view the Country of incident. |
| Classification | Allows the user to view the attachment classifications which is associated to the case. |
| Description | Allows the user to view the attachment description which is associated to the case. |
| Rejected Date | Allows the user to view the date when the case was rejected. |
J Literature Book-in
In Japan, the Drug Safety receives literature data from JAPICWMDIS (World Medical & Drug Information Service). This data includes a literature list of company-related drugs. When the safety division receives this information , it assesses the content and determines reportability for each type of literature on the list.
Import Tab
In Argus J, you can import these literature data files and assess reportability from the loaded data. The system enables you to book-in all necessary data. Select Literature Intake from the Worklist menu to open the New Literature Case Intake Worklist screen.
The following table lists and describes the fields on the New Literature Case Intake Worklist screen.
| Field/Control Name | Description |
|---|---|
| Import Date Range | Enables you to import literature data for a specific time period. |
| Start Date | The beginning date for the import. |
| End Date | The end date for the import. |
| Range | The Range field of the data. |
| Search | Click Search to filter the import items. |
| Total # of Rows | The number of rows that display on a page. |
| Displaying Rows | The rows that the system currently displays. |
| Page Size | The number of rows that the system is configured to display on each results page. |
| Import Date | The date the literature data is loaded to the system from the shared folder. The date is in YYYY/MM/DD format. |
| Literature Type | The type of literature to display. Right=click to select the type to display. |
| Search Equation Number | The unique value associated with a specific search. |
| Document Number | The unique value that identifies a specific document. |
| Product Family | The produc family name associated with the Search Equation number or the name that has matching ingredients with the WMDIS data.
The system displays all ingredients from WMDIS literature data inside a particular product family.
|
| Ingredients | This column displays individual ingredent length. |
| Title | The title of the piece of literature. |
| Author | The name of the person who wrote the literature. |
| Journal | The name of the journal the literature was published in. |
| Vol. | The volume number of the issue the literature was printed in. |
| Issue | The number that identifies the specific issue of the journal in which the literatue appeared. |
| Page | The page the data starts on. |
| Publish Year | The year the journal was published. |
| Screening | This column contains the screening results. It displays the one of the following:
|
| Assigned to | Displays the name of the assigned user, if applicable. |
| Add Individual Information | When clicked, the system enables you to add individual literature information. |
| Print List | Enables you to print the list of data. |
When using the Import tab, be aware of the following:
-
You can filter all column items.
-
Tabulation and sorting functions are available for your use.
-
All Worklist basic functions are available to you.
-
You can use a background process to import.
-
The system permits you to import both JAPIC and WMDIS data.
-
If the data format does not match the JAPIC or WMDIS formats, the system does not import the file and places it in the Failures fold with the error description files.
-
The system assumes the contents are valid.
-
-
The system imports the data and stores it in a shared table.
-
The system does not validate the data content.
-
The system can import .xls and .xslx files for WMDIS.
-
When the system imports literature data, it sorts the data by date in descending order (newest at the top).
-
If there are multiple items with the same date, the system sorts them by internal sequence number.
-
The following right-click options are available in any row:
-
Accept -- Enables you to accept a highlighted. When clicked, the system displays "Accepted" in the Screening column.
-
If a product Family is not specified for the selected item, the system opens the Modify Product family dialog box to enable you to select a valid product family.
-
When an item is marked as accepted and assigned to a use, the system moves the data item to the Proccessing tab and saves the user name and date time stamp (GMT) in the source file.
-
If the system does not assign a user for an item, the data remains on the Import tab until a user is assigned.
-
-
Reject -- enables you to reject the highlighted item. When clicked, the system displays "Rejected" in the Screening column.
-
When you reject an item, the system opens a Justification dialog box, to enables you to enter or select a justification for rejecting the item.
-
Rejected by the Standard Screening is the default value in the dialog box.
-
Once an item is rejected, the system moves the data item to the Processed tab and saves the user name and date time stamp (GMT) in the source file.
-
-
Assign User -- Enables you to assign another user to be the owner of the data item. The system opens the ''Reassign" dialog to enable you to assign an owner.
-
Assign Literature Type -- Enables you to specify the Literature Type from the Console J Codelist. When clicked, the system opens the popup so you can assign an owner.
-
Modify Product Family -- Enables you to define a product family for the literature when a Product Family is not available.
-
Print Monitoring Check Report -- Enables you to print the Monitoring Check Report form in PDF format.
-
Multiple Accept -- The Multiple Accept option enables you to accept all selected row items.
-
When the system encounters any accepted items with a Product Family, it displays the following error message: "At least one product family is missing from the selected items. Only items with a product family are marked "Accepted."
-
If the system encounters locked records, it displays the following message: "Data is being used by another user. The system cannot process this data.
-
-
Multiple Reject -- The Multiple Reject option enables you to reject all selected items.
-
When you reject an item, the system opens the Justification dialog box to enable you to enter a reason for rejecting the item.
-
The default value is "Rejected by the Standard Screening."
-
The justification you enter in this dialog box applies to all selected items.
-
If you are a trying to process locked records, the system displays the following message "Data is being used by another user. The system cannot process this data."
-
-
Assign Multiple Users -- The Assign multiple users option enables you to assign users for the assessment in the Processing tab for all selected rows.
-
Assign Literature Type to Multiple Items -- The Assign Literature Type to multiple items option enables you to specify the literature type for all selected rows.
-
Literature Duplicate Search -- Enables you to perform a duplicate search.
The following table lists and describes the fields on the Duplicate
-
| Field/Control Name | Description |
|---|---|
| Title | Displays the title of the current search item. |
| Vol. | Displays the volume number associated with the current search item. |
| Issue | Displays the issue associated with the current search item. |
| Page | Displays the number of the page where the current search item appears. |
| Publish Year | The year the current search item was published. |
| Search from Literature Intake | When selected, the system executes the search from the Literature Intake Import tab, Processing tab, and Processed tab.
This is the default. The system executes the search on all Import, Processing, and Processed tabs. Howeve , the search result does not include the original search item. |
| Search from the Case Data | When selected, the systm executes the search from the case data. |
| Search | Click Search to start the search operation. |
| Reject Literature Information | When clicked, the system removes the selected line from the imported list. |
| Close Duplicate Search | Cancels the search operation and closes the Duplicate Search screen. |
| Total # of Rows | The total number of rows in the search results. |
| Displayed rows | The total number of rows currently displayed. |
| Page Size | The maximum number of data rows that appear on a page. |
| Search Equation Number | The unique value that identifies the search operation. |
| Document Number | The unique numeric identifier assigned to a selected piece. |
| Ingredients | A list of ingredients discussed in the literature. |
| Title | The title of the piece of literature. |
| Author | The author of the article. |
| Journal | The name of the Journal in which the article appeard. |
| Vol | The volume number associated with the journal in which the article appeared. |
| Issue | The issue of the journal in which the article appeared. |
| Publish Year | The year the article was published. |
| Status/Case Number | The status of the data. This can be one of the following:
|
| Screening Owner | The user name of the person who first accepted the imported literature. |
| Assessment Owner | The user name of the person who performed the second assessment. |
When performing a duplicate search, be aware of the following:
-
A duplicate search searches for title, vol, issue, page and publish year and is executed in the Processed imported literature date and the Case Data.
-
By default, the system searches processed data.The system enables you to search for case data by selecting a radio button. When you do so, the system displays the data in the table at the bottom of the screen.
-
You can modify field content and click the sesarch button as in E2B Duplicate Search.
-
You can use the % character to perform a partial search.
-
When the system displays the search result, it enables the Reject Literature Information button if it finds at least one duplicate. When you click the button, the system marks the original item "rejected."
-
If the result set contains more than 1000 items, the system only displays the first 1000 items.
-
When you execute a duplicate search from the Case Data tab, the columns that do not appear in the Case data (Search Equation Number, Document Number, Issue, Screening Owner, and Assessment Owner, are left blank.
-
The system displays the generic name for the ingredients in the Case Data.
-
The system displays Author J, Title, J, and Journal J in Japanese. If Japanese values are not availble, the system displays the English values.
-
When you click "Add Individual Information" button, the system enables you to enter data about an individual piece of literature. The system opens the Add Individual Information dialog box.
-
When you click Select, the system opens the Literature Lookup dialog to enable you to select literature from the Code list.
-
When you click Add, the system adds the entered nformation to the Import table.
-
When you click Cancel, the system closes the section.
Processing tab
The Processing tab enables you to provide information for processing literature data.
The following table lists and describes the fields and controls on the Processing tab.
| Field/Control Name | Description |
|---|---|
| Date Range | Enables you to enter a range of import or screening dates. |
| Start Date | The date to start the search. |
| End Date | The date to end the search |
| Range | The range field for the data. This is the value from the Date Range code list item. |
| Search | Click Search to initiate the search. |
| View Individual All | Enables you to view a single piece of data or all retrieved items. |
| Total # of rows | The total number of rows in the search results. |
| Displaying rows | The total number of rows that are displaying. |
| Page Size | The total number of rows to displays on a single page. |
| Import Date | The date the literature is loaded into the system from the shared folder. |
| Literature Type | The selected literature type. |
| Search equation number | The unique value that identifies this search query. |
| Document Number | The unique value that identifies a specific document. |
| Ingredients | The list of ingredients referenced n the piece of literature. |
| Title | The title of the article. |
| Author | The author of the article. |
| Magazine Name | The name of the publication where the article appeared. |
| Vol | The vol of the magazine in which the article is published. |
| Issue | The specific issue in which the article is published. |
| Page | The page on which the article appears. |
| Publish year | The year the article was published. |
| Assessment | Enables you to view the result of the second assessment (when available).
When you click Assessment, the system opens the Justification dialog box. |
| Owner | The assigned owner of the literature. |
| Print List | Enables you to print the list. |
The following right-click options are available in any row:
-
Assessment
-
Assessment Result
-
Multiple Assessment
-
Create Case/Process Unnecessary Assessment
When you select Assessment from the drop-down list, the system opens the Justification dialog box.
The following table lists and describes the fields on the Justification dialog box.
| Field/Control Name | Description |
|---|---|
| Assessment Result | This drop-down list enables you to select the assessment result you want to view. |
| Possibility of occurrence of serious disease such as cancer, disorder, or death | Enables you to choose whether to include assessments where there is a possibility of serious disease. |
| Significant change on event or infection occurrence number, frequency and condition. | Enables you to choose whether to include assessments where there has been a significant change in and event or condition. |
| Doesnt' have acknowledged effectiveness | Enables you to choose whether to include assessments where there hasn't been any acknowledged effectiveness. |
| Problems | Enables you to enter information about any problems. |
Assessment Result drop-down list
The Assessment Result drop-down list includes the following items. The selection you make determines what is mapped to the case form.
| Item | Description |
|---|---|
| Not Necessary | Select this item to move the data item to the Processed tab after the "Create Case/Process Unnecessary Assessment" action. |
| AE Case | Select this item to move the data item to the Processed tab after the "Create Case/Process Unnecessary Assessment" action. |
| Research/Infection Report (Marketed Drug) | Select this option to move the data item to Analysis/PMDA General/Reporting Category. |
| Research/ADR Report (Marketed Drug | Select this option to move the data item to Analysis/PMDA General/Reporting Category. |
| Research/Infection Report (Investigational Drug) | Select this option to move the data item to Analysis/PMDA General/Reporting Category. |
| Research/ADR Report (Investigational Drug | Select this option to move the data item to Analysis/PMDA General/Reporting Category. |
| Research Report (Quasi Drug) | Select this option to move the data item to Analysis/PMDA General/Reporting Category. |
| Research Report (Cosmetics) | Select this option to move the data item to Analysis/PMDA General/Reporting Category. |
| Measures in foreign countries including discontinuation of manufacture, recall and withdrawn (Marketed Drug) | Select this option to move the data item to Analysis/PMDA General/Reporting Category. |
| Measures in foreign countries including discontinuation of manufacture, recall and withdrawn (Investigational Drug) | Select this option to move the data item to Analysis/PMDA General/Reporting Category. |
Selecting this option enables you to assess all selected row items.
Create case/process unnecessary assessment
When you select this option the system does the following:
-
If "Assessment" for the Literature data is marked "not necessary," the system moves the data to the Processed tab.
-
If Assessment for liter data is marked with any of the other options, the system opens the Book-in screen with all the literature data populated. The system copies the assessment result to Case Data.
-
When you select "Create case/Process unnecessary assessment," the system verifies there is a valid Japanese license available to the product family. If the product family doesn't have a Japanese license, the book-in fails and the system displays the following message: "This product family does not have a valid Japanese license."
-
If a matching product family does not exist at the time of the book-in, the system does not populate the product on the book-in screen.
-
If an assessment is not completed for the literature data you select "Create Case/Process Unnecessary Assement," the system displays teh following message: "Assessment result does not exist."
-
When booking is complete, the system presents the following message: "New Case <case name> successfully created. Do you want to open the case?"
-
Once the booking is complete the system moves the processed item to the Processed tab.
Processed tab
The Processed tab enables you to provide information about processed literature data.
The following table lists and describes the fields and controls on the Processing tab.
| Field/Control Name | Description |
|---|---|
| Date Range | Enables you to enter a range of import or screening dates. |
| Start Date | The date to start the search. |
| End Date | The date to end the search |
| Range | The range field for the data. This is the value from the Date Range code list item. |
| Search | Click Search to initiate the search. |
| Total # of rows | The total number of rows in the search results. |
| Displaying rows | The total number of rows that are displaying. |
| Page Size | The total number of rows to displays on a single page. |
| Import Date | The date the literature is loaded into the system from the shared folder. |
| Literature Type | The selected literature type. |
| Search equation number | The unique value that identifies this search query. |
| Document Number | The unique value that identifies a specific document. |
| Product Family | The product family referenced n the piece of literature. |
| Title | The title of the article. |
| Author | The author of the article. |
| Magazine Name | The name of the publication where the article appeared. |
| Vol | The vol of the magazine in which the article is published. |
| Issue | The specific issue in which the article is published. |
| Page | The page on which the article appears. |
| Publish year | The year the article was published. |
| Screening | Enables you to view the screening results. |
| Date | The screening date. |
| Owner | The assigned owner of the literature. |
| Assessment | Enables you to view the assessment results. |
| Date | The assessment date |
| Assigned | The user name of the person who performed the assessment. |
When using the Processed tab, be aware of the following:
-
The grid only displays accepted literature data.
-
The Status Detail field opens when you highlight a single item.
Enter general information
The General tab captures the case information in categorized sections for category-specific information. It also enables you to enter or view information such as the type of report, literature information, and so forth.
-
If a priority has not been assigned to a case, the Case Priority field is hidden.
-
If a case owner has been assigned to the case, the name of the case owner appears at the top of the Case form.
General information—field descriptions
| Field/Control Name | Description |
|---|---|
| Report Type | Select a report type that determines the availability of fields relating to clinical studies and literature references.
The Clinical Study report prompts for information relating to the study, and literature-based reports enable to select the journal and reference on which the case is based. |
| Country | Select the country where the adverse event occurred. |
| Initial Receipt Date | Enter the date your company became aware of the case. Argus Safety uses this date throughout all reports.
This date can be changed only prior to regulatory report submission. |
| Central Receipt Date | Enter the date on which this information was received by Central Safety. |
| Medically Confirm | Specify whether the case was medically confirmed or not, by a healthcare professional. |
| Initial Justification | Enter the initial justification reason.
The entry in this field is displayed as per the reason entered when the case is being booked in. |
| Amendments/Follow-ups | On sorting by Follow-up received dates, the serial number still displays the order of entering the follow-ups. The follow-up information can be sorted by:
Click the header to sort in ascending or descending order. By default, the sorting is in descending order of the Follow-up Received Date. |
| Follow-up Received Date | To enter the date on which amendment or follow-up information was received by your company, click Add.
You can select whether the case has significant follow-up information by confirming the message: Is this follow-up significant?. When sorting on follow-ups, by default, the dates are sorted in descending order. |
| Safety Received | Enter the date on which follow-up information was received by Central Safety. This field is disabled for an amendment and any prior data in this field is cleared. |
| Significant | Check this checkbox if the follow-up is significant. |
| Data Clean up | Check this checkbox to mark the follow-up as a Data Clean up version.
This version is used in the Data Lock Point for Case Versioning in and System Reports. This field is disabled for an amendment and any prior data in this field is cleared. |
| Amendment | Specify if the case was updated without adding any new information.
This checkbox is enabled irrespective of whether the Significant checkbox is checked. |
| Amendment/Follow-up Justification | Select a pre-defined justification for Amendment or Follow-up.
Click the icon to view the standard justifications dialog box. The justification entered in the Amendment field is also populated in this field. You can select a pre-defined justification from this dialog box or enter a new justification. |
| Case Requires Follow-up | Check this checkbox if the case requires follow-up information. |
| Classification | Select up to 50 case classifications used to categorize a case.
To enter additional case classifications, click Add. When the Classifications field is hidden, the classification section is hidden on the Case Form |
Enter study information
-
To choose from the available list of study information, click Select.
-
In the Clinical Trial Selection dialog box, enter Project, Study, and Center information.
The Clinical Trial Selection dialog box allows you to select a clinical trial from the list configured by the administrator.
-
Click Search.
Tip:
To broaden the search results, enter as little information as possible. Select the required clinical study and study center, and click Select. -
Choose the study information from the list, and click Select.
The details of the selected Study Information are added to each field in the Study Information section.
Study information—field descriptions
| Field/Control Name | Description |
|---|---|
| Project ID | Enter the Project ID, or select one from the list.
Selecting a Project ID automatically creates items in the Study ID list. |
| Study Phase | Enter the Study Phase for the configured study.
This field is pre-populated if you select a Study with an already-configured Study Phase. |
| Blinding Status | The status is auto-populated based on the type of study.
You need special access rights to use any of the Broken By entries. Note: The Unblind Case dialog box appears when you try to unblind a study. For Not Blinded studies, saving the case or generating a report, you can enter the actual drug (vs. placebo) given to the patient. |
| Observe Study Type | The value selected from this drop-down list is populated in the Case Form Study Section when the Clinical Study is selected. |
| Unblinding Date | Auto-populates when the blinding status is changed to 'Broken by Sponsor' or 'Broken by Investigator'.
If you double-click the date in this item, the Unblind Case dialog box is displayed. If the date of unblinding is more recent than the date for the most significant follow-up information, an automatic follow-up is generated. The unblinded date is not editable in the Unblind case dialog box when blinded status is changed to 'Broken by Sponsor' whereas it is editable when the blinded status is changed to 'Broken by Investigator'. |
Enter reporter information
Enter the information about the person providing the case-related information.
-
The Reporter Rearrangement dialog box displays the number of Reporters present in the case. It displays the First Name and Last Name, followed by the Reporter Type in brackets, as entered in the reporter information dialog box.
-
To view all the Reporters, click the Quick Launch icon.
-
To view the details of the selected Reporter tab, click a Reporter Name.
-
To add a new reporter, click the New tab. You can add a maximum of 100 reporters.
-
The Primary Reporter is identified by the Reporter icon on the Reporter Information tab.
Add reporter information
-
In the Reporter Information section, click Select.
-
In the Reporter Lookup dialog box, enter the search criteria, and click Search.
-
All filter criteria you have entered on the Reporter Look Up dialog box are saved as user preferences while it populates the reporter information on the General tab.
-
If you have reporter information in the case, the information appears in the Reporter Lookup dialog box and search is performed.
-
After performing the search the search criteria is retained as user preferences. The next time you perform a search, these preferences appear.
-
When you log out, the user preferences are retained and are available the next time you log in.
-
To clear all the values in the filter elements, click Clear.
-
Tip:
You can choose to search either by Search Cases or by Search List Maintenance. -
-
From the search results, select the reporter information, and click Select.
The selected, pre-defined information is added to the fields.
Reporter information—field descriptions
| Field/Control Name | Description |
|---|---|
| Reporter Notes | Click this button to enter free text notes relating to this reporter.
This field supports multiple language entries. Click a flag, select the language tab, and enter information. |
| Institution ID | Enter the reporter's institution ID.
This field value is populated based on the Institution ID selected for the Reporter from the Reporter Lookup dialog box. This field also allows to manually enter or update the value directly in the Case Form irrespective of the value specified in the Institution field for the reporter. Manually entered Institution and Institution ID field values are allowed in the Reporter Information section even if they are not specified or linked to each other as per Console Institution code list. |
| Protect Confidentiality | If this checkbox is checked, the name and address of the reporter does not appear on regulatory reports and the reporter's information displays "NAME AND ADDRESS WITHHELD".
The 'MSK' null flavor appears in the eVAERS report when this checkbox is checked. MSK is populated only when the data element contains some data and is not null. |
| Primary Reporter | Only one primary reporter is permitted per case. The primary reporter is the reporter whose name appears on the regulatory reports. The tab that identifies the primary reporter is displayed in blue as compared to the other reporter tabs. |
| Correspondence Contact | If this checkbox is checked for a reporter, the reporter's address information is used in letters. You can select more than one reporter as the correspondence contacts for the case. |
| Reporter ID | If known, enter the Reporter ID. This completes the Case Form reporter fields. |
| Reporter Type, Report Media, and Intermediary | These lists are maintained by the administrator. |
| (New) Tab | Creates details for a new reporter. |
J Reporter Lookup dialog box
The following table lists and describes the fields in the J Reporter Lookup dialog box shown in the following illustration.
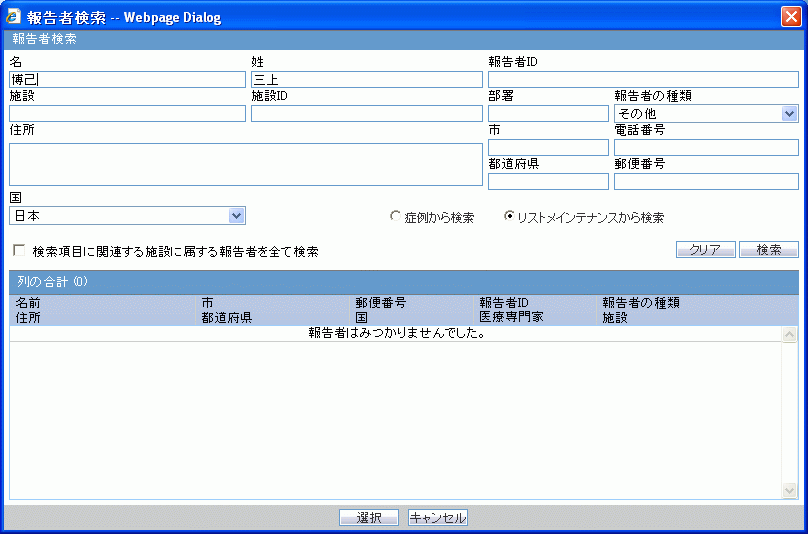
| Field/Control Name | Description |
|---|---|
| First Name | Enter the first name of the reporter. |
| Last Name | Enter the last name of the reporter |
| Reporter ID | Enter the reporter's ID |
| Institution | Enter of select the institution. |
| Institution ID | The unique value that identifies the institution. |
| Reporter Type | Enables you to select the reporter type from the drop-down list. |
| Address | Enter the address of the reporter. |
| City | Enter the city of the reporter. |
| State/Province | Enter the state/province of the reporter. |
| Postal Code | Enter the postal code of the reporter. |
| Phone Number | Enter the phone number of the reporter. |
| Country | Enables you to select the reporter's country. |
| Search Cases | Click this button to search for cases that match the specified search criteria. |
| Search List Maintenance | Click this button to search the list maintenance for the specified criteria. |
| Search all the reporters who belong to the institution found from the current serch item | When checked, enables you to search for all reporters linked to the specified institution. The result list shows all the reporters belonging to the institution
When you click this checkbox the system does the following:
|
When using the Reporter Lookup dialog box, be aware of the following:
-
The "Search all the reporters who belong to the institution found from the current search item" checkbox is enabled only when the "Search List Maintenance" radio button is selected.
-
You can select multiple reporters in the search results table. Hold down the Shift key and click each item to select it.
-
When this option is enabled, the system places the multiple reporter information in the Case Form when you click Select.
-
When you click Select, and one or more reporters are already in the Case Form, the system adds these reporters on the Case Form.
-
When you select multiple reporters, the information replaces the current tb and adds a new tab for each additional report.
Enter literature information
You can enter a literature reference for a case manually or you can enter pre-defined literature information.
-
From the list of available literature, click Select.
-
In the Literature Reference dialog box, select a reference in the list, and click Select.
The details of the selected literature information are added to each field in Literature Information.
Enter patient information
You can enter patient information such as the patient's past medical history and current conditions, and laboratory tests and test results.
Enter current medical status
-
In the Case Form > Patient tab, click Current Medical Status.
-
From the items in the form, select the options that apply to the patient.
If you don't know whether a particular condition applies for the patient, select Unk.
-
To save the current medical status, click OK.
Copy patient information from reporter information
If the patient and the reporter are the same person, the reporter information entered in the General tab can be copied to the Patient tab.
To copy the reporter information, click Patient Info From Reporter.
Patient information—field descriptions
| Field/Control Name | Description |
|---|---|
| Child only Case | If this checkbox is checked, then the pregnancy [Detail] button is accessible from the Parent information tab only and no longer through the Patient tab directly. |
| Current Medical Status | Captures details about the history and the current condition of the patient from the dialog box.
The items that appear in the Current Medical Status dialog box are mapped to the fields for the German BfArM tab on the Analysis tab. Changing the values on the BfArM tab does not affect these items. |
| Name
(Title, First Name, MI, Last Name) |
During book-in, the patient name is transferred to the relevant name and initials fields. If the Patient Name or Initials are three characters or less, this is transferred to the Initials field. |
| Number of Patients | Enter the number of patients involved in the adverse event. |
| Pat. ID | Enter the Patient Identifier number.
Note: This field appears for clinical trial cases only. Tip: This field can be used while searching for cases in the Case Selection dialog box. |
| Protect Confidentiality | If this checkbox is checked, the patient's name and address will not appear on any of the regulatory reports and the patient's information will show the word PRIVACY.
The 'MSK' null flavor appears in the eVAERS report when this checkbox is checked. MSK is populated only when the data element contains some data and is not null. |
| Randomization # | Determines which drug was administered to the patient during the course of the study.
Note: This field appears for clinical trial cases only. Tip: This field can be used while searching for cases in the Case Selection dialog box. |
| Sponsor Identifier | Enter the Sponsor Identifier of the patient.
Note: This field appears for clinical trial cases only. Tip: This field can be used while searching for cases in the Case Selection dialog box. |
Enter patient notes
Click ![]() to enter free text notes relating to the Patient. If there is no text data for Patient Notes, from the NF drop-down list, select Null Flavor.
to enter free text notes relating to the Patient. If there is no text data for Patient Notes, from the NF drop-down list, select Null Flavor.
Note: This field supports multiple language entries. Click a flag icon to enter data in different languages.
![]() Icon is displayed when there is no data in Patient Notes and NF data.
Icon is displayed when there is no data in Patient Notes and NF data.
![]() Icon is displayed when there is data in Patient Notes or NF data.
Icon is displayed when there is data in Patient Notes or NF data.
Enter patient details
Enter patient information, including pregnancy data.
Table 2-1 Patient Details—Field Descriptions
| Field | Description |
|---|---|
|
Ethnic Group |
Enter the patient's ethnic group such as Hispanic or Latino, Not Hispanic or Latino, and so on. |
|
Military Status |
Enter the details of the Military status of the Patient such as Active Duty, Reserve, National Guard, TRICARE Beneficiary, and so on. |
|
Race |
Select the patient's race. You can capture up to 5 Race using the Add and Delete buttons. Note: You cannot select the same Race more than once. |
Enter pregnancy information
-
From the Pregnant drop-down list, select Yes.
This field is shown as active only after the Gender field in this section is selected as Female.
The Pregnancy Information section appears.
-
Enter the available pregnancy information in the form.
-
Click OK.
-
In the Neonate Information section:
-
To delete neonate information, right-click the neonate, and click Delete.
-
The changes are tracked to the neonate information in the audit log.
-
The following list describes the fields on the Pregnancy Information and Neonate form.
| Field/Control Name | Description |
|---|---|
| Gestation Period | Enter the gestation period when the reaction or event was observed in the fetus. Select a unit for the gestation period from the accompanying drop-down list. |
| Number of Fetus | Enter data for a baby who is born to the patient.
To make an additional entry, click New. |
| Prospective/Retrospective | Select if the information was Prospective or Retrospective.
A prospective information is one where the company hears of the case before the baby is born to the patient who took the drug. In a retrospective case, a company gets to know after the baby is born. |
Enter patient death details
Enter information about the death of a patient.
Event death details—field descriptions
| Field/Control Name | Description |
|---|---|
| Autopsy Done? | If Autopsy Done is set to No or Unknown, the Autopsy Results Available is shown as No. |
| Autopsy Results Available? | The Autopsy Results Available? field is enabled only if the Autopsy Done? field is marked as Yes.
If Autopsy Results Available? is changed to No, and Autopsy Result rows exist, you will be asked to delete the rows first. Click Yes to delete the data. |
| Add | Click this button to add a Cause of Death and Autopsy Results row.
Note: You can add multiple records, up to 50 entries. |
| Delete | Deletes a highlighted row. |
Enter other relevant history
Enter information related to past drugs administered on Patient, and Current Medical condition of the patient.
To copy the Other Relevant History rows, select the row, and click Copy.
Other relevant history—field descriptions
| Field/Control Name | Description |
|---|---|
| Copy | Enables you to copy a row. After you copy the row, the focus will be on the newly copied row. |
| Add | Enables you to add a row to the relevant history. After you add the row, the focus will be on the new row. |
| Delete | Enables you to delete a row from the relevant history. |
| Start Date | Enter the start date of the condition. You can enter a partial date if the actual date is not available. You can also choose not to enter a date.
Note: If you click Add but do not enter a date, the Date column is removed. Once the date is entered and a test is associated with the date, you cannot clear the date but can only modify it. To remove the date column, individually delete all the cells in that Date column. |
| Stop Date | Enter the stop date of the condition. You can enter a partial date if the actual date is not available. You can also choose not to enter a date.
If you select Ongoing as Yes or Unknown, the Stop date is cleared and disabled. |
| Age and Units | Age and Age units are enabled only for condition type with Patient Other Relevant Therapy being set. |
| Family History | Check this checkbox if this relevant history is reported to be present in another family member. The Family History checkbox is enabled only for Condition types that are mapped to ’Medical History Episode' in the Condition Type codelist. |
| Substance Information | Displays the substance name, term ID, and strength unit of the Product separated by commas. If there are multiple records, they are displayed in subsequent rows. |
| Name Part | Displays the name part and name part type information. If there are multiple records, they are displayed in subsequent rows.
Click Name Part button to enter Product Name Part Type and Product Name Part details. |
| Product Name Parts Information | Displays the Product Name Part information. Enabled only for condition type with Patient Other Relevant Therapy being set. |
| Version | Displays the Date or Version of the Product Identifier. Enabled only for condition type with Patient Other Relevant Therapy being set. |
| Coded PT/Description of condition LLT/Indication PT/Reaction PT | Enter a term to describe the condition. You can either manually encode or auto-encode if you have been so configured by the Administrator.
In manual mode, type the description (for example, Fever). In Auto-encode mode, enter a partial description and press ENTER or TAB. The appropriate coding dialog box appears. In either mode, click Encode and modify the encoding. If the condition type is historical drug, the encoding will be done with WHO drugs. |
| Product Identifier Type/ Product Identifier Version/ Notes | Displays the type of Product Identifier such as MPID, PhPID, and so on. Enabled only for condition type with Patient Other Relevant Therapy being set.
Displays the Date or Version of the Product Identifier. Enabled only for condition type with Patient Other Relevant Therapy being set. |
| Encode | Click this button to encode the term. Encode is used in multiple contexts such as coding with MedDRA for reactions and indications, and coding for products using WHO drug browser.
Note: To view the complete MedDRA hierarchy for the encoded term, click the encoding status icon. |
| Indication PT and Reaction PT | This field is visible on the case form only if the condition type selected in the List maintenance is Patient Other Relevant Therapy. |
| Ongoing | Indicates whether the condition is continuing. If it is set to Yes or Unknown, the Stop date is disabled. |
Enter lab data—lab test and test results
The maximum number of lab test data on the Case Form is 1500.
-
To enter a Lab Test Name:
-
Click Add Test.
-
Enter the description, and click Search.
-
From the search result, select a name.
-
-
To select a Lab Test Group, click Select.
A list of the lab test that matched the selected group appears.
If lab test data is already in the case form, the lab test group is appended after the last lab test.
-
To add more rows of Lab Test Data, right-click an empty cell, and click Add.
-
To copy a Lab Test Data, right-click the row to be copied, and click Copy.
-
To delete a Lab Test Data, right-click the row to be deleted, and click Delete.
-
To arrange entries in a specific order, click the Order icons.
-
You can sort Lab data in chronological order by Date of the Test and alphabetically by the Test Name.
If there are partial dates entered, the date is displayed at the beginning of the month, and year for the date entered.
-
To view the hierarchy of the Event Term, click the view icon.
-
To view Notes in a Zoom dialog box, click the view icon.
-
To arrange the Lab Test Data, click the arrow button to move data to left or right.
This is available only when the Lab Test is entered for the same date.
Lab data—field descriptions
| Field/Control Name | Description |
|---|---|
| Norm Low/Norm High | The Test Name list can retrieve details of the normal range for the test selected (if the administrator has entered the normal range into the list). Otherwise, enter the values manually. |
| Result/Units | Enter the test result, including units, and select a term to describe the qualitative assessment of the results. The administrator can modify the list of possible assessments.
If test results cannot be expressed in Result/Units or Assessment, enter the data in Notes field. You must enter Lab test result by using Results/Unit, Assessment or Notes fields, and not in all the fields. Regulatory agencies may send rejection for ICSR if data is sent in all formats. |
| Assessment | Select a qualitative assessment term to describe the lab test results. |
| More Info Available | Check this checkbox if there is additional information is available for this Lab test in Additional Info tab. |
Enter product information
Enter and view details for products and dosage regimens. This tab contains the name of the drug that has been entered here. For Blinded Studies, the Blinded Product Name field appears in the tab.
If you do not have access to view unblinded information on the Case Form, some of the fields are hidden.
If the study has been unblinded and a study drug had been selected, the selected Study Drug Name is displayed. You cannot view unblinded information and the tab continues to show the Blinded Product Name.
You can enter details of more than one product and more than one dosage regimen for a company product for which multiple licenses exist (for example, drug and vaccine, or drug and device).
Based on the type of license (drug, vaccine, or device), different views are available in the Products tab. If the selected item is not a company product or if a license for a company product does not exist, all three views are always available.
Search for products
Product Browser based search
-
In the Products tab, click Select.
The Product Browser dialog box appears.
-
Click the displayed entities.
The hierarchy above and below the entity being searched is also displayed. For example, if Product Name is searched, it displays the Product Name as well as the Family Name and Trade Name.
-
Search for Products based on the following criteria:
-
Ingredient
-
Family
-
Product Name
-
Trade Name—Searches the License Trade Name
-
-
To select all the search criteria, select the Full Search checkbox, and click Select.
-
Select a product from the search result.
-
To remove the entered search criteria, click Clear.
WHO Drug Browser based search
-
To open the WHO Drug Coding dialog box, click Encode.
-
From the Case Form Configuration dialog box where the Dictionaries are selected for encoding, select either the WHO Drug B format or the WHO Drug C format.
-
Enter the search criteria, and click Search.
You can search for a product based on:
-
Trade Name
-
Formulation/Strength (sequence 3 and sequence 4) of the product
-
Country—The Sales Country Code of the Product as defined in the WHO Dictionary
-
Generic.
The following criteria are not available for display or searching in the WHO Drug B Format:
-
Formulation
-
Country
-
Strength
-
Generic
-
Medicinal Product ID
-
Product Type
-
-
-
To copy the selected drug to the Product tab, click Select.
-
To close the selection dialog box without making any updates to the Product tab, click Cancel.
-
To perform a full search from the WHO Drug browser, select Full Search.
-
By default, a like search is performed (e.g., CUREALL%)
-
You can use the percent (%) sign to perform wildcard searches
-
If you click Full Search, the full search is performed (e.g., %CUREALL%)
-
-
You can also search for drug formulation and country. This option is available only if you select the WHO Drug C format.
-
To clear the search criteria you entered, click Clear.
-
You can sort the results on all the fields.
J Drug Browser
When using the J Drug browser, be aware of the following:
-
Clicking Enclode enables the Drug Coding (J-Drug) dialog box.
-
The system store both the WHO and the J codes.
-
The system uses the WHO drug code in the English screen for global cases.
-
You can search for a product by typing the product name in the Description field and/or entering the drug code in the Code field and clicking Search.
-
The system searches the J Diction and returns a list of products in Japanese character order.
-
The system places a list of products from J Drug in the "Proprietary J-Drug" list box. Scroll through the list and click a product to select it. Click Select to choose the product used for the case.
-
When you click Select, the system closes this screen and populates the following fields in the Case Form:
-
Product Name
-
Generic Name
-
J Drug Code
-
Enter time measurement information
You can enter seconds in the following fields:
-
Argus > Case Actions > Open > (Select a Case) > Event tab > Event sub tab > {event description} sub tab > Event Information section (middle of screen)
-
Onset From Last Dose field
-
Duration field
-
Onset Latency field
-
-
Argus > Case Actions > Open > (Select a Case) > Products tab > Product sub tab > {Product Name} sub-sub tab (drug) > Dosage Regimen section (lower 1/3 of screen)
-
Duration of Regimen
-
-
Argus > Case Actions > Open > (Select a Case) > Products tab > Product sub tab > {Product Name} sub-sub tab (drug) > Product Details section (lower 1/3 of screen
-
Duration of Administration
-
Time between First Dose/Primary Event
-
Time between First Dose/Primary Event
-
-
Any number following by the letter "s" defaults to "#sec."
-
The seconds entered in the form are interpreted in the following formats, where:
# is a number from 0 to 9
#s—changes the format to # sec.
# s—changes the format to # sec.
# sxx
where:
x is other letters—that changes the format to # sec
-
The Temporal View and the Case Form printout display the seconds.
-
The E2B import and export case functions support seconds and M2 Validation for the defined fields.
Enter drug information
Product information—field descriptions
This section enables you to enter information about the drug being used for the case.
| Field/Control Name | Description |
|---|---|
| Product Information | |
| Product Name | Enter the name of the product using the Select button or by entering a partial product name. Type a partial product name and press TAB. This displays the Product Selection dialog box.
If only one product is found, this information is entered without showing the dialog box. If no match is found in the company product list, the WHO Drug Dictionary is searched for a possible match through the WHO Drug Dictionary dialog box. If a match is still not found, the text you initially typed in, is used as is. Note: If the study is blinded, the Blinded Name of the clinical study is displayed in this field. After unblinding, the selected study's Product Name for Unblinded cases is not shown. |
| Select | Displays the product selection dialog box.
Select a product from the list of company products click Select. The relevant fields are added to the Case Form. |
| Encode | Click Encode to retrieve the code. |
| Suspect/ Concomitant/ Treatment | Make a selection for the product you are entering. The drug types indicate the involvement of the product with the adverse event(s) reported for the case.
Suspect indicates that the product may have caused the adverse event(s). Concomitant indicates drugs that are taken with the suspect drug. Treatment is the drug taken to treat the adverse event. |
| Generic Name | Enter the generic name of the drug in a manner similar to the Product Name. If the study is blinded, the Generic Name is replaced with the Study Name of the product.
Note: This name is entered based on the selected company product. If the study is blinded, the Blinded Name of the clinical study is displayed in this field. After unblinding, the selected study's Product Name for Unblinded cases is not shown. |
| Product Identifier | Displays the type of Product Identifier such as MPID, PhPID, and so on. |
| OTC Product | Check this checkbox if the product administered was an over-the-counter (OTC) product. |
| Compounded Product | Check this checkbox if the product administered was compounded. |
| Company Drug Code | Displays the licensed country for the selected company product. |
| Obtain Drug Country | Country the drug is licensed in. |
| Drug Code | Enter the WHO-DRUG code. |
| WHO Medicinal Product ID | Displays the Medicinal ID associated with the selected WHO drug. The WHO Drug code used to identify the drug.
Note: This ID is populated only if a WHO-drug is selected. |
| Formulation | Select the formulation of the product. The form in which the drug was administered (liquid, tablet, capsule, and so on.)
Note: This field is entered based on the product. Contact your administrator to adjust this list. |
| Drug Authorization Country | The country where the drug is licensed. |
| Market Authorization Holder | Captures the name of the Marketing Authorization Holder of the product that was administered. |
| Authorization Type | Captures the Authorization Type of the product that was administered such as IND, BLA, PLA, and so on. |
| Authorization Number | Captures the Authorization Number or License Number of the product that was administered. |
| Manufacturer | A different Manufacturer can be selected from the drop-down list and can still be kept as a company product. |
| Concentration | Indicates the amount of the drug that was administered.
After a drug and formulation have been entered, select the concentration from the list, or enter the concentration. If this information is changed manually, the product is marked as a non-company product. Note: This field is entered based on the chosen product. The concentration cannot be modified for a Study drug. |
| Interaction? | Indicates whether the case involves a drug interaction. |
| Contraindicated? | Indicates whether the drug was administered contrary to its indication. |
| Drug Not Administered | Check this checkbox if no drug was administered on the patient. |
| Substance Information | |
| Substance Name | Displays the Ingredient's name. |
| Strength | The Strength of the substance used in the Product. |
| Units | The drug unit (such as mg, tsp, and so on). |
Note:
Drug Authorization Country, Authorization Type, Authorization Number, and Marketing Authorization Holder data are populated from the Argus Console for company products.For Study Products, the system populates the Authorization Type, Authorization Number, and Marketing Authorization Holder data based on the Primary license number selected in the Argus Console > Studies Configuration. When unblinded at the case level, Authorization Type and Authorization Number data is not accessible for users with restricted access to unblinded data.
Enter product indications
Enter information about the indicator of the adverse event, like:
-
Reported Indication—Reported reaction
-
Coded Indication—Code for the reaction
Both the values could be different or same.
Table 2-2 Product Indication—Field Description
| Field/Control Name | Description |
|---|---|
|
Reported Indication |
By default, the value of this field is populated with the Product Indication from the Product Configuration, if set up. If not, you can enter a term into the Reported Indication field. Note: Argus Safety automatically encodes this information. You can also click Encode to open the coding dictionary dialog box. |
|
Coded Indication |
This field is populated with the encoded term when you enter data in the reported indication field and tabs out. |
|
Encode (Indication) |
Opens the MedDRA Browser with the term already populated from the Coded Indication field. To view the complete MEDDRA hierarchy for the encoded term, click the Encoding Status icon. |
|
Add |
Adds a new Indication row. Only two indications are visible at a time |
|
Delete |
Click this button to delete the selected Indication row. |
Enter Quality Control information
To enter quality control information, click Quality Control.
When you enter information in this section, an action item is automatically created in that case with text Case Product <<Product Name>> sent for QC requires follow up. This action item is assigned to the you.
If you do not intend to create any action item, then it add a date in the Product > Quality Control > QC Result Date field.
The Action item code when QC info is entered common profile switch is used to set the action item code as selected in this switch. If this switch is left blank, then no action item code is set.
| Field | Description |
|---|---|
| Quality Control Safety Date | Enter the Quality Control department reference number for the analysis. |
| Quality Control Cross Reference | Enter the Quality Control department reference number for the analysis. |
| # CID Number | Control Identification Number |
| PCID Number | Product Control Identification Number |
| Lot Number | If the Lot Number entered is incorrect, a Lot Number Lookup dialog box is displayed, that allows you to select from the existing lot numbers. |
| Quality Control Result Date | Enter the date the result of the analysis was received by the Quality Control department. |
| Quality Control Result | Enter a text explanation as necessary. Use the zoom icon to open the zoom notes and view or edit the text. |
Enter dosage regimens
| Field/Control Name | Description |
|---|---|
| Stop Date/Time | Enter the stop date and time of the dosage. Entry of time information is optional, and you can enter partial dates.
Note: If no Stop Date is entered, Onset from Last Dose is calculated from the Event Onset Date and the most recent Stop Date or the most recent Start Date. |
| Ongoing | Select this checkbox if the drug treatment is ongoing.
The Stop Date, Duration of Regimen, and Last Dose fields are removed if this checkbox is checked. |
| Outside Therapeutic Range | Check this checkbox if the drug has not been used in accordance with the label or has been used for outside the Therapeutic Range.
Contact your administrator for further company-specific information on the use of this field. |
| Duration of Regimen | This value is calculated based on regimen start and stop dates (if full dates are entered for the start and stop dates). If the value is entered manually, the duration units (for example: minutes, hours, days, months, or years) must also be entered along with the actual duration.
Note: Contact your administrator to set the duration to be inclusive or exclusive. In Inclusive mode, the starting day counts in the calculation of the duration; in Exclusive mode, it does not. |
| Dose Description | This value is entered by using the values from Dose, Dose Units, and Frequency. If necessary, you can change this value.
If Dose, Units, or Frequency information is changed, this value will be recalculated. |
| Daily Dosage | This value is calculated based on the dose and frequency. It can be manually overwritten. If either the dose or the frequency fields are blank, this field is not calculated automatically. |
| Regimen Dosage | This value is calculated based on the daily dose, duration, and frequency. This total can be overridden. If the daily dose is blank or the frequency fields are 0, this field is not calculated automatically. |
| Regimen Dosage Unit | This value is derived based on the Daily Dose Units. |
| Accidental Exposure | Select the type of Accidental Exposure from the list. A non-modifiable list of items is provided for this list. |
| Pack Units | Select the package presentation information of the product.
Contact your administrator to adjust this list. |
| (New) Tab | Click this tab to create a new dosage regimen entry. |
Enter product details
| Field/Control Name | Description |
|---|---|
| First Dose | The earliest regimen start date. |
| Last Dose | The latest regimen stop date. |
| Duration of Administration | Calculated automatically if full dates are available for the first and last doses. |
| Total Dosage | Calculated based on daily dose and duration. |
| Time Between First Dose/Primary Event | The time from the first dose to primary event onset. |
| Time between Last Dose/Primary Event | The time from the last dose to primary event onset. |
| Total Dose to Primary Event | The cumulative dose to the time of the event. |
| Action Taken | Select a term.
If you select Dose Increased or No change, the dechallenge and rechallenge fields are disabled. Contact your administrator to adjust this list. |
| Dechallenge Results | Indicates the drug stopped for the purpose of determining if it was the drug that caused the adverse event. |
| Date | Enter the date the dechallenge was carried out. |
| Gestation Period at Exposure | Gestation Period at Exposure is automatically calculated for all Products in the case by using the formula: Date of First Dose of the respective Product – LMP Date. It is calculated only if full dates available for both fields. |
| Taken Previously / Tolerated | Select a response from the list. |
| Rechallenge Results | Make a selection based on whether the drug was taken again.
If Pos or Neg or UNK is selected for the Rechallenge field, the following fields are enabled:
|
| Start Date/Time | Enter the date and/or time when the rechallenge was started. |
| Stop Date/Time | Enter the date and/or time when the rechallenge was stopped. |
| Specialized Product Category | Select specialized FDA product categories such as combination products, compounded and repackaged products used in eVAERS reports. |
| Abuse | Check if the patient abused the product (For example: Painkillers taken without pain). |
| Batch and lot tested and found within specifications | Check if the batch and lot was found to be within specifications, after being tested. |
| Batch and lot tested and found not within specifications | Check if the batch and lot was not found to be within specifications, after being tested. |
| Tampering | Check if the product appeared to have been tampered with before it was used. |
Enter study drug information
You can enter a Study Drug for a Non-Configured Study entered in a case and mark a current product as a study drug.
To mark a product as a study drug:
-
Right-click any suspect product in the case.
-
To mark the current product as a study drug, select Make Study Drug.
Make sure:
-
The drug type is disabled to make the product a Concomitant or Treatment option.
-
Study Drug is a read-only field that contains the product name selected by you.
-
For non-configured studies in the case, the following message appears for all study drugs in the case:
Drug Not Administered.
Enter device information
Enter product information
| Field/Control Name | Description |
|---|---|
| M/W Info | The MW Info dialog box allows you to enter the following device information:
|
|
|
|
|
| EU / CA Device | The EU/CA Device dialog box allows you to enter the device information. Fields are marked by either an EU flag or a Canadian flag to indicate which entity is mapped to the field.
|
Enter product indications
Select product delivered by device
| Field/Control Name | Description |
|---|---|
| Type of Drug | Select the type of drug from the drop-down list. |
| Other Mfg Product | Displays the Other Manufacturing Product. |
| Add | Adds another row. |
| Delete | Deletes the highlighted row. |
Enter device component information
| Field/Control Name | Description |
|---|---|
| Component Name | The device component name used in the product. |
| Component Term ID | The device component term ID of the device component used in the product. |
| Version | The Date or Version of the device component term ID of the device component used in the product. |
| Batch Lot # | The batch lot number of the device component name used in the product. |
| Add | Adds another row. |
| Delete | Deletes the highlighted row. |
Device information—field descriptions
-
You can enter up to 10,000 characters in the Additional Manufacturer Narrative field.
-
The device evaluation codes have been updated to reflect FDA standards. Go to the following link for more information:
http://www.fda.gov -
The evaluation method is available is the following site: http://www.fda.gov
-
The evaluation results are available at the following site: http://www.fda.gov
| Field/Control Name | Description |
|---|---|
| Product Information | |
| Catalog # | Under Catalog#, enter the exact catalog number as it appears in the manufacturer's catalog, device labeling, or accompanying packaging. |
| UDI System | The UDI System captures the unique identification type of the given device.
The valid values are: GS1 – Global Standards 1 HIBCC - Health Industry Business Communications Council ICCBBA - The International Council for Commonality in Blood Banking Automation |
| Unique Identifier (UDI) # | Under Unique Identifier (UDI) #, enter any other applicable identification number (for example: component number, product number, part number, bar-coded product ID).
Data is entered in such a way to handle the UDI Device identifier and UDI Production Identifier fields. |
| Operator of Device | Select the type of person operating or using the suspect medical device on the patient at the time of the event such as Health Care Professional, Patient, Paramedic and so on. |
| If other | If the operator of the device is other, enter the operator of the device. |
| Malfunction Type | Make a selection to indicate the type of reportable event. For an event associated with a malfunction, the FDA refers to applicable sections in 21 CFR Part 803 reporting guidelines. |
| Device Available for Evaluation | Indicate whether the device is available for evaluation. Also, indicate whether the device was returned to the manufacturer and if so, the date of the return. |
| CE Marked | Select whether the device is CE Marked or not. This information is preloaded from License information for a case booked manually, through E2B or LAM, and can be modified as needed. |
| Malfunction Information | - |
| Reported Malfunction | Enter the malfunction as entered by the reporter. |
| Determined Malfunction | Enter the malfunction as determined by the company. |
| Listedness | Enter the listedness of malfunction in respect of the device. |
| Reportable | Select the reportability of the malfunction. |
| Incident Information | This section captures the incident details. It contains the following fields and supports multi-record entry. The Event Type Level codes are cascading, that is, Event Level 2 depends on 1.
Event Type Level 1 Event Type Level 2 Manufacturer Event Type Level 3 Event Type Level 3 Description |
| Manufacturers Final Investigation Results | This section captures the investigation details. It contains the following fields and supports multi-record entry. The Evaluation Type Level codes are cascading, that is, Evaluation Level 2 depends on 1.
Evaluation Type Level 1 Evaluation Type Level 2 Manufacturer Evaluation Type Level 3 Manufacturer Evaluation Type Level 3 Description |
EU/CA device dialog box—field descriptions
| Field/Control Name | Field Length | Field Type |
|---|---|---|
| NCA Reference Number | 100 characters | Alphanumeric |
| Identify to what other NCA's this report was also sent | 2000 characters | Alphanumeric |
| Number of Patients Involved | 3 characters | Numeric |
| Number of Devices | 3 characters | Numeric |
| User facility reference number | 20 characters | Alphanumeric |
| Remedial Action by HC Facility | 1000 characters | Alphanumeric |
| Usage of Medical Device | N/A | Checkbox |
| Other | 15 characters | Alphanumeric |
| Update to Initial Report (Follow-up Report) | N/A | Checkbox |
| Final Report | N/A | Checkbox |
When you select the EU/CA Device option, the fields on the Case form are printed. A track of the fields and any updates are maintained in the audit log.
Enter vaccine information
Enter product details
This section is the same for the Product Details section under all the tabs - in the Drugs tab, Device tab, and the Vaccine tab.
Enter prior adverse events information
-
Enter the relevant vaccination information items in the form.
-
Click OK.
Enter product information
| Field/Control Name | Description |
|---|---|
| Include in VAERS Block | Select the VAERS Form block that this vaccine needs to be printed in. |
| Suspect/Concomitant/Treatment | Make a selection for the product you are entering. The drug types indicate the involvement of the product with the adverse event(s) reported for the case.
|
| Generic Name | Enter the generic name of the drug in a manner similar to the Product Name. I
if the study is blinded, the Generic Name is replaced with the Study Name of the product. This name is entered based on the selected company product. |
| Formulation | Select the formulation of the product. Contact your administrator to adjust this list.
Note: This field is entered based on the product. |
| Drug Authorization Country | Displays the licensed country for the selected company product. |
| Concentration | After a drug and formulation have been entered, select the concentration from the list, or enter the concentration. If this information is changed manually, the product is marked as a non-company product.
Note: This field is entered based on the selected product. The concentration cannot be modified for a Study drug. |
| Units | Select a concentration unit.
Contact your administrator to adjust this list. |
| Interaction? | Indicates whether the case involves a drug interaction. |
| Contraindicated? | Indicates whether the drug was administered contrary to its indication. Make the appropriate selection to indicate whether the drug was contraindicated in this case. |
Complete vaccine administration form
| Field/Control Name | Description |
|---|---|
| For VAERS Form-1 Use Only | |
| Resp. Physician | Enter the name of the physician responsible for the patient. |
| County | Enter the county where the patient was vaccinated. |
| State | Enter the state where the patient was vaccinated. |
| CDC/FDA VAERS # | Enter the verification number. |
| Purchased With | Select an item from the list to describe how the vaccine was purchased. |
| Vaccine Facility Information | |
| Facility Name | Enter the name of the facility where the vaccine was administered. |
| Country | Enter the country of the facility where the responsible physician works. |
| Facility Type | Enter the facility type where the patient was vaccinated. |
| Facility Military Flag | Indicates whether or not the vaccination facility was a Military facility. |
Enter vaccine history
| Field/Control Name | Description |
|---|---|
| Route of Admin | Enter the route of administration or a short code for the route of administration.
Contact your administrator to adjust this list. |
| Anatomical Location | Select the anatomical location of the vaccination.
Contact your administrator to adjust this list. |
| Block 14 | If this box is checked, this Vaccine History will be printed in the VAERS Form Block 14. |
| Add | Adds a new Indication row.
Note: Only two indications are visible at a time. |
| Delete | Click this button to delete the selected Indication row. |
Enter event information
You can enter or view details for adverse events for a case using this tab. It has the following sub-tabs:
-
Events tab
-
Event Assessment tab
-
Product-Event Details tab
Events tab—field descriptions
| Field | Calculation |
| Total Dosage | The sum of all Total Regimen Dosages. |
| First Dose to Onset | The system calculates this value based on the First Dose Stop date and the event onset date if present.
If any regimen start date is null, the system sets the First Dose to Onset to null. The system uses only complete date entries for the regimen start and stop date/time fields to calculate First Dose to Onset. The user can overwrite this field. |
| Last Dose to Onset | The system calculates this value based on the last dose stop date and the event onset date if present.
If the regimen start date is null, the system sets the Last Dose to Onset is to null. The system uses only complete date entries for regimen start and stop date/time fields to calculate the Last Dose to Onset. The user can overwrite this field. |
-
You can change the listedness for a drug at the individual level.
-
The field labels for the Event Assessment tab can be updated and configured on the Argus Console.
-
If the Death Seriousness criteria on the Event tab are unchecked, the event outcome reverts to empty and is not set to fatal.
-
The MedDRA LLT term selection behavior across the Case Form is based on the profile switch value configured in the Allow User to Add Non-Current MedDRA Terms for and On change of LLT Term Sync English and Japan LLTs, irrespective of the currency.
-
The system populates the To be coded value on the MedDRA pop-up window when you check the green checkbox for the MedDRA hierarchy. This applies to the areas where the MedDRA dialog box appears.
-
The Event to Exclude from Report field enables you to identify information to not include in a PMDA Expedited report.
-
When this checkbox is checked, enter a justification for this action in the Reason to Exclude from the Report dialog box.
The system places a symbol to the right of the field.
-
The system does not retrieve events that are excluded from a report as part of CSPSR (Clinical Study Periodic Safety Report) unless it has been configured to include them.
-
When you click the Recalculate button, the system does not recalculate listedness where the Event Assessment Listedness already has a case justification (generated automatically or manually overwritten).
Enter event information
The Event information tab lets you encode adverse events, record criteria for event seriousness, and display results of automated assessments that determine whether events are listed in data sheets. It also lists the licenses for the data sheets.
Event information—field descriptions
| Field/Control Name | Description |
|---|---|
| Relationships | Click the Relationships button to view the Diagnosis-Event Relationships dialog box. This allows you to group symptoms and signs with diagnoses. This requires at least two events and a diagnosis. |
| Description as Reported | Enter the verbatim term used by the reporter, or patient, to describe the adverse event. As you type, the term is copied under Description to be Coded. Verbatim English translations of foreign languages may also be entered here. |
| Term Highlighted by Reporter | Indicate whether the primary source reporting the event considered it a major concern. If the information was not explicitly provided, the term is not considered a highlighted term. The seriousness of the reaction/event must be based on the ICH E2A criteria.
Selecting Yes does not mark this case as Serious. |
| Description to be Coded | You can modify the verbatim term in this field. For example, the original term can be split, or enhanced with an anatomical location. Contact your system administrator to prevent manual encoding of the description. If automatic encoding is enabled, the term can be auto-encoded to the included term level. |
| Onset Date/Time | Enter the date/time when the event started. Enter a partial date if the full date is not available. |
| Onset from Last Dose | This field is calculated from the event onset date and most recent stop date listed in the dosage regimen details of the suspect drug(s). You can also enter or modify the field manually. This field is removed if the dosage regimen is ongoing. |
| Duration | This field is calculated from the event start and stop dates. You can also enter or modify the duration manually. |
| Onset Latency | This field is calculated from the earliest first dose date of the suspect drug(s) to onset date. You can also enter or modify the duration manually.
Onset Latency = Onset Date - First Dose. |
| Receipt Date | Enter the date on which information about this event was received by your company. In Flexible Aggregate Reporting, this field is used to filter the events that fall out of the reporting period. |
| Patient Has Prior History? | Indicate whether the patient has had a prior history or has suffered from the same event in the past. |
| Intensity | Select the category of severity of the event. Contact your system administrator to adjust this list. |
| Frequency | Select the frequency of the event from the list. Contact your system administrator to adjust this list. |
| Outcome of Event | Select the outcome of the event. Contact your system administrator to adjust this list. If Fatal is selected, Death is selected in the list of seriousness criteria.
If the Death checkbox is subsequently cleared, the outcome still remains fatal. |
Review a diagnosis-event relationship
You can group events in a case, and/or associate them with particular diagnoses. Your company may determine these or they may be reported to it. This helps in interpreting and reviewing individual case reports. You can also enable the reporting of diagnoses only while retaining database records of individual event terms.
Click the Relationships button in the Event Information section to open the Diagnosis-Event relationship dialog box.
The case must contain at least two events and, at least, one diagnosis. The events related to a diagnosis are listed on top in this dialog box and the symptoms are indented to the diagnoses. You can group events together and associate them with individual diagnoses.
Associate a symptom with the diagnosis
Click the up or down arrows to associate a selected symptom with a diagnosis. In Argus Safety, select the symptom and click Move Up or Move Down.
For example:
Defining a diagnosis-event relationship can clarify an adverse event report. Suppose an initial case report describes a patient suffering from Somnolence, Sore Throat, and Fever.
AE # Diagnosis AE Term (Associated AEs)
1 Somnolence
2 Sore Throat
3 Fever
For reports on all events, they would appear on a CIOMS-I form as:
-
Somnolence [SEDATION]
-
Sore Throat [SORE THROAT NOS]
-
Fever [PYREXIA]
Suppose a Follow-up report then supplies information that the patient also had neutropenia, and had been diagnosed as suffering from agranulocytosis (the cause of the sore throat and fever). The somnolence was considered to be coincidental, and unrelated to any other adverse events.
Neutropenia and agranulocytosis would be entered into the system, and a diagnosis-event relationship established as follows:
| AE # | Diagnosis AE Term (Associated AEs) |
|---|---|
| 1 | Yes agranulocytosis |
| 2 | (sore throat) |
| 3 | (fever) |
| 4 | (neutropenia) |
| 5 | Somnolence |
These events would appear on a CIOMS I form as:
-
AGRANULOCYTOSIS
-
[AGRANULOCYTOSIS] [SORE THROAT],
-
[PYREXIA NOS], [NEUTROPENIA]
-
Somnolence [SEDATION]
This immediately provides a clear clinical picture of the case.
Auto-populate event information
The MedDRA application searches the term dictionary for a match at the Lower level or at the Synonym level. If a match is found, the following fields are automatically populated: Term code, Preferred Term, Included Term, High Level Term, Group Term and Body System/SOC.
Configure regulatory reporting rules
The regulatory reporting rules are mainly configured to look at Seriousness, Listedness, Causality, and Outcome. Out of these, Listedness and Causality can be captured and controlled (using the event assessment section) down to an individual license basis.
This granularity allows individual license holders to override the normal listedness and causality assessment to control the need for submissions to their local regulatory authority. Each affiliate could either suppress the need for a report by demoting the criteria or add the requirement for a report by promoting the listedness or causality.
This serves to promote the global reporting automation while maintaining the level of individual local affiliate control that is often needed.
To obtain an assessment of the adverse event, the product must be in the company's suspect product and the event must be encoded.
Enter event coding information
The Event Coding section enables you to enter information about the event.
| Field/Control Name | Description |
|---|---|
| System Organ Class (SOC) (Code) | Displays the body system or System Organ Class. This item is automatically entered from the event dictionary used and cannot be edited. |
| High Level Term (Code) | Displays the high-level group term when using the MedDRA coding dictionary. This field is not shown when coding with WHO-ART and cannot be edited. |
| High Level Term (Code) | Displays the High Level Term when the MedDRA coding dictionary is used. This item is not displayed when event encoding is done using the WHO-ART dictionary and cannot be edited. |
| Preferred Term (Code) | Displays the preferred term. This item is automatically entered from the event dictionary used and cannot be edited. |
| Included Term (Code) | Displays the lower level term code. This item is entered from the event dictionary used and cannot be edited. |
Enter seriousness criteria
| Field/Control Name | Description |
|---|---|
| Death | Displays the Death Details dialog box
Note: If you un-check the Death option in the Seriousness Criteria, you are required to confirm the deletion of the death details. |
| Hospitalized | Displays the Event Hospitalization dialog box. |
| Other | Check this checkbox to enter explanatory text. You must mandatorily enter text, specifying the Other Seriousness Criteria. |
Enter death details
-
Check the Death checkbox under Seriousness Criteria.
The form for Event Death Details appears.
-
Enter information for the items in the form.
-
Click OK to save the entered Death Details.
Note:
When Seriousness criteria Death is unchecked, the system displays a message Do you wish to delete the Death Details?. On confirming, the Death date (NF or date) and Autopsy details are cleared.Enter hospitalization details
-
Check the Hospitalized checkbox under Seriousness Criteria.
The form for Hospitalization Details appears.
-
Enter information for the items in the form.
-
Click OK to save the entered Hospitalization Details.
Enter event assessment information
The Event Assessment tab enables you to capture causality and listedness information for a case. All encoded events in the case are compared with the listed events for company products (agents) associated with the case.
Event assessment tab—user actions
| User Action | Result |
|---|---|
| Click Datasheet column's icon | The system does the following:
|
| Click Product Name link | Displays the name for the selected product. |
| Click Event Description link | Displays the Event Information for the selected event. |
| Click Datasheet Description link | Displays the first 1000 terms in the datasheet. Click Print to view all the terms and click Export to export all the labeled terms to an Excel file in a single column with column header as Labeled Terms. |
Event assessment—field descriptions
| Field/Control Name | Description |
|---|---|
| Recalculate | Refreshes the Event Assessment section with the newly entered data if new suspect products or events are entered, or the Event Relationship is modified. |
| Product | Populated when events are entered in the Products tab and appears as:
|
| Causality as Reported/Source/ Method/ Result | Provides the source of causality as reported such as Investigator, Healthcare Professional, and so on.
Provides the method used for causality determination. Provides the result of the causality assessment. |
| Causality as Determined/Source/ Method/ Result | Provides the source of causality as determined such as Sponsor, MAH, and so on.
Provides the method used for causality determination. Provides the result of the causality assessment. |
| Other Causality /Source/ Method/ Result | Provides the source of causality from any additional source such as NCA.
Provides the method used for causality determination. Provides the result of the causality assessment. |
| Event | Populated when events are encoded and appears as:
|
| D/S | Displays the Diagnosis/Symptom details by D or S. |
| Seriousness Severity Duration | Displays the seriousness, severity, and the duration of the event. |
| Listedness | Indicates whether the system found the event on the datasheet for this product. |
Filter event assessment details
Only the assessment rows that match the selected criteria appear in the filtering results.
| Field/Control Name | Description |
|---|---|
| Product | The Product filter drop-down list contains all products listed in the event assessment. You can filter on all the products which are present in the Event Assessment dialog box. |
| Event PT(Description)/LLT | Contains a drop-down list of values of distinct Event PT. You can filter on all the products which are present in the Event Assessment dialog box. |
| D/S | Contains a drop-down list of values of D for Diagnosis or S for Symptoms. |
| Datasheet | Contains a drop-down list of values of distinct Datasheets. |
Enter product-event details
The Product-Event Details tab enables you to capture causality information for a case.
| Field/Control Name | Description |
|---|---|
| Event | Populated when events are encoded and appears as:
|
| Onset from First Dose | This date is defined as the earliest regimen start date to Onset and is completed automatically. The date calculation is based on the Case Form Calculation for Inclusive or Exclusive. |
| Onset from Last Dose | This date is defined as the latest regimen stop date to Onset and is completed automatically. The date calculation is based on the Case Form Calculation for Inclusive or Exclusive. |
| Total Dose to Event | This value is calculated based on daily dose and duration. |
Events Tab: Product -- Event details tab
The Product -- Event Details tab enables you to enter information for an adverse event related to a specific product. The following is an illustration of the Product -- Event Details tab.
Product -- Event Details tab fields and field descriptions
You can enter data in the following fields at a Product Event combination level:
-
Most important diagnosis?
-
Event more specific/severe than PT?
-
Action taken
-
Other text
-
Onset from First Dose
-
Onset from Last Dose
-
Total Dose to Event
-
Units
-
De-challenge Results
-
Re-challenge Results
-
Event Occurred as a Consequence of
-
Term
Be aware of the following:
-
By default, all the new fields are hidden in the Event Assessment. If all the fields are hidden, the system also hides the Product-Event details tab.
-
You can enter data in the Event Occurred as Consequence of field from the type ahead.
-
When you select the Event Occurred as Consequence of field, the list of corresponding values configured in the Argus Console is available for the user to select from the Term list.
-
You can add or delete elements in the Event Occurred as Consequence of fields.
-
You can enter a maximum of 20 entities in the Event Occurred as Consequence of field. After the user enters 20 entities, the system disables the Add button.
-
-
Filtering for Product. The product Filter drop-down list contains all products listed in the event assessment.
-
You can filter on products on the Event Assessment dialog.
-
By default, the system displays all the products with the <ALL> option.
-
When filtering, the system displays only the assessment rows that match the product you selected.
-
-
Filtering for Event. The Event Filter contains a drop-down list of values of distinct events.
-
You can filter on events in the Event Assessment dialog.
-
By default, the system displays all events with the <ALL> option.
-
When filtering, the system displays only the assessment rows that match the events you selected.
-
-
The Product Event details are tied to the Event Assessment section. You can access these details only if your user group permissions give them access.
-
The system tracks case updates in the audit log.
-
The system prints the additional fields on the Case Form print out. The additional fields are linked to the Event Assessment section of the case form printout.
The following table lists and describes the fields on the Product -- Event Details tab.
| Field/Control Name | Description |
|---|---|
| Product | Enables you to select a product whose event details you want to review. |
| As Reported Causality/As Determined Event PT (Description)/LLT | Enables you to view details about a specific adverse event if more than one is associated with this product. |
| Most Important Diagnosis? | Enables you to specify whether this is the most important diagnosis. |
| Event More specific/severe that PT? | Enables you to indicate whether treatment more serious than PT was required for this adverse event. |
| Onset from First Dose | The amount of time from the first dose was taken until the time the adverse event occurred. |
| Onset from Last Dose | The amount of time from the last dose was taken until the time the adverse event occurred. |
| Total Dose to Event | The total number of doses until the event occurred. |
| Units | The amount of each dose given to the patient. |
| Action Taken | Enables you to enter the action taken when the adverse event occurred. |
| Other | Other information related to the adverse event. |
| Dechallenge Results | ~ |
| Rechallenge Results | ~ |
| Event Occurred as a Consequence of | The cause of the adverse event. |
| Term | ~ |
| Add | Click Add to add another row. |
| Delete | Click Delete to delete a specific row. |
Add attachments to your case
The Additional Information tab lets you attach notes and other items to a case. For example, you could attach a fax message that came in as part of the case and needs to be scanned and attached or an electronic file received by email. It also lets you set up cross-references to other cases such as links between cases referring to mothers and children. The total number of attachments and references attached to a case appears in the header.
-
When you click the hyperlink and a reference case is present, the system opens a case number irrespective of the selected reference type when you clicked the hyperlink.
-
If no sites are defined for the attachments classification, the system permits all users to view the attachments on the Additional Information tab.
-
The system permits Workflow Enterprise to view all attachments across all sites.
-
You can send different Case Form attachments to different agencies, based on the Attachment classification specified to the Receiving Agency in the Reporting Destination Codelist in the Argus Console. For more information, refer to the Argus Safety Administrator's Guide.
-
After the records in Notes and Attachments are sorted, if the following options are invoked without closing the case, the sort order is respected in these modules:
-
Case Form Print
-
Medical Review
-
Copied Case
-
Enter additional information for the attachments
| Field/Control Name | Description |
|---|---|
| Notes and Attachments | |
| Notes and Attachments | Lets you sort column headers to sort the records based on the fields in the Notes and Attachment section. |
| Classification | Select a classification that describes the attachment. Contact your system administrator to adjust this list.
Refer to the Oracle Argus Interchange User's Guide for details about Attachment Classification usage in sending attachments in electronic reports. |
| Incl. Reg. Sub | Check this checkbox to merge PDF attachments within the Case Form. The Incl. Reg. checkbox identifies the attachments to be included as an appendix to Expedited reports. On checking this checkbox, the Expedited reports - CIOMS I, CIOMS I (Local), US FDA MedWatch 3500 Drug and 3500 A Device print an appendix page before each attachment. This is added to the case, and marked as Inc. Reg. Sub checked.
The checkbox is available only if a PDF is selected as an attachment. |
| Literature Reference | Select the Literature that is related to the attachment. Attachments that have Literature reference are transmitted as inline attachments for the data element C.4.r.2 in the E2B(R3) report. |
| Attach File | Inserts a file attachment into the case. Maximum file size is 4 GB. |
| Attach Documentum Link | This option is visible only if Documentum is available.
Click Searching for Documentum to search for and attach Documentum. |
| References Type | Select a reference type from the list, for example, a parent-child link. Contact your system administrator to adjust this list. |
| ID # | Enter the case number of the case that is to be referenced.
Click Select to search for the case that is to be referenced. You can also use this field to record the reference number for external cases. |
| Select | Opens the Case Selection dialog box for the selected ID. |
Sort attachments
| Field | Description |
|---|---|
| Keywords | On clicking this column header for the first time, records are sorted in the ascending order (alphabetically) of the Keywords. |
| Date | On clicking this column header for the first time, records are sorted in the ascending order (chronologically) of the Date. |
| Description | On clicking this column header for the first time, records are sorted in the ascending order (alphabetically) of the Description. |
Search for Documentum links
-
Click the Attach Documentum Link button to open the Documentum Lookup screen.
-
Enter the desired search criteria as per Type Name, Attribute Name and Search String, and click Search.
-
Select the desired link from the row displaying the search results.
-
Click Select to select the link from the list.
Attach files to a case
-
In the Notes and Attachments section of the Additional Info tab, click Attach File to open the Attachment dialog box.
-
Click Browse to locate a file attachment.
-
Select the file and click OK.
Enter keywords
You can associate keywords with a case in the Notes and Attachments section.
To attach keywords to a case:
-
Go to the Notes and Attachments section and click Select.
-
When the system opens the Attachment Keywords dialog box, select a keyword from the Select a keyword to add to the list drop-down list.
The system displays the selected keywords in the Keywords field.
-
Click OK
Attach references to a case
-
Locate the References section and click Select.
-
When the system opens the Case Search Criteria dialog box, enter the search parameters and click Search.
-
When the system displays the search results in the Total Number of Rows section, select the desired search criteria from the list and click Select to view details about the selected case.
View and print attachments
The Attachments tab enables you to view and/or print case attachments. The system prints date/time information:
-
As footers on all printouts (except letters).
-
In the following format: dd-mmm-yyyy hh24: mm: ss.
To print case attachments:
-
Click the Description link to display the attachment.
-
When the system opens the letter, click Print to print it.
Review local labeling
-
Hover over the Worklist menu and select Local Labeling.
-
When the system opens the Local Labeling screen, select the appropriate information as necessary.
-
Filtering by Product Family: You can filter products in the Event Assessment dialog box based on the selected product families. Click the magnifying glass icon to filter the search results by product family.
-
The system displays the number of cases currently in view and automatically updates the range based on the page size specified in the Search dialog box (read-only). For example, if you select 100, the system divides the displayed rows into groups of 100 cases.
-
Events assessment can show all listedness values. By default, it shows listedness only for the core datasheets and countries you have permission to access.
-
Diagnosis -- The Diagnosis Filter contains a drop-down list with the following values:
-
D (Diagnosis)
-
S (Symptoms)
-
In the Events Assessment dialog box, you can filter on either the diagnosis or the symptom.
-
-
By default, the system displays all events with the <ALL> option.
-
Datasheets
-
The Datasheets drop-down list contains a list of distinct datasheets.
-
All the blank datasheets display as a single row of Unspecified.
-
When you click the Datasheet hyperlink, the system displays the datasheet notes.
-
-
Licenses
-
The Licenses drop-down list contains a list of distinct countries for the licenses.
-
All licenses not associated with a datasheet appears under Unspecified and are aligned with the datasheet view.
-
When you click the Licenses hyperlink, the system displays the license references.
-
-
The Process button triggers all the applicable rules (both global and local) for the country/license type for the licenses that are assessed through this screen.
For more information about Local Labeling, see the LAM User Guide for Worklist - Local Labeling requirements.
Enable local data entry for Japan
Argus Safety supports a concept of global lock that indicates the readiness of case data for global reporting, and a concept of local data locks that indicates the readiness of case data for local reporting having fulfilled the local data entry and assessment needs.
Enable local data entry
The application allows local users to open a case for entering local data without globally unlocking the case and at the same time maintain the integrity of the "global" case data.
In order to achieve this:
-
The fields in the application are categorized into global and local fields. The local fields have been identified as local to one or more countries. The local fields for only Japan are supported in this release.
-
All Argus J users are considered as Japan Local user in this release and as having access to edit the Japan local fields.
About local fields
A field is identified as a Local field to one or more countries based on a new attribute in the CMN_FIELDS table. The local fields for only Japan are supported in this release.
Customers can configure any field in the Case Form > General, Patient, Products, Events tabs, and in the Analysis > PMDA tab as a Local field for Japan through back-end updates to the CMN_FIELDS table.
The Enterprise copy configuration action will copy these updates as per existing functionality.
If a customer configures a field that could update global value as a local field, it is expected that the customers maintain the integrity of the global data by business SOPs or custom software processes.
All the fields in a case that is not a Local PRPT case are simply treated as global fields.
All Argus J users are considered as having access to edit the Japan local fields.
All Japanese text fields, including J User-Defined fields are considered as Local fields for Japan.
i. All Japanese text fields are the fields that currently has separate _J columns.
ii. All fields from PMDA tab, PMDA Device Information section.
Argus Safety allows selection of secondary LLT (stored in LLT_J or LLT_CODE_J field) or Synonym (SYN_CODE_J) encoding using MedDRA J browser that does not change the base MedDRA hierarchy of English.
The Event Assessment > Listedness field in the tab for the licenses of the local country corresponding to the local user is considered as a local field. However, this field is available for editing for the user only when the local user has listedness privilege for that local country assigned to them via the User Group > Listedness Determination - Countries access and the datasheet associated with that local country license is configured with "Global / No Local Assessment Required" checkbox as unchecked.
The Events tab > Infection and Event Exclusion checkbox are also considered as a local field for Japan.
Any field where an update to a global field can occur is NOT considered as Local field.
All numeric fields, date fields, drop-down fields which share same data value for English and Japan sides are not considered as Local fields.
However, there are exceptions to this rule where some fields that contain global values are available for update to local users and in such a scenario, it is expected that the global value should be protected by customer's business SOPs.
Case classification is such a field that can cause an update to the global value and it is available as a Local field in the application out-of-the-box.
Study section under General tab requires special handling by the application during local editing. Study Name, Study Description, Protocol Number, Clinical Compound Number and Center Name are local fields.
When the study is a configured study, all these fields are disabled for local editing except Center Name (J). Center Name (J) is available as an editable local field.
Product Information section in Products tab requires special handling by the application during local editing. J Drug Code type and the corresponding J Drug Code/OTC Drug Code/Temporary Code (i.e., DRUG_CODE_TYPE_J, DRUG_CODE_J,), J Generic Name and J Product Name fields are local fields.
Any field that is already editable after case lock will remain editable even after local lock.
An auto-narrative generation performed during Japan Local data entry (after global lock) only updates the J field value and does not update the English or any other language field value.
Access local case data lock functionality
This section lists the different sections where the functionality for Local Case Data Lock has been documented in the Argus Safety suite of documentation.
Refer to the following table for the list of features and the corresponding Guides where they have been documented:
| Local Lock Feature | Overview | Documented in |
|---|---|---|
| Local Locking and Local Unlocking - Configuration | Introduction of new switches: Allow Local Locking - to allow a local user to be set up with the privilege to locally lock or unlock a case
Enable Local Unlocking - to provide a system level control permitting local users to locally unlock a case and make any corrections to the previously entered local data. |
Oracle Argus Safety Administrator's Guide > 2 Access Management > Configuring Users
Oracle Argus Safety Administrator's Guide > 4 System Configuration > Configuring System Management - Common Profile Switches Oracle Argus Safety User's Guide > Global User Management |
| Changes to the Case Locking Mechanism in Argus Safety - Case Form Changes | Introduction of action icon - Local Lock - to allow a user to locally lock or unlock a case | Oracle Argus Safety User's Guide > 1 Getting Started > Quick Launch Toolbar |
| Changes to the Case Locking Mechanism in Argus Safety - Changes to global locking, One Step Global and Local Lock | Allow Global locking and triggering of global and local reports, and allow one step global and local locking | Oracle Argus Safety User's Guide > Locking a Case |
| Changes to the Case Locking Mechanism in Argus Safety - Changes to Global Unlocking - Configuration | Introduction of new switches:
Allow Forced unlock (Global and Local) - to allow users to be set up with the privilege to forcibly unlock a case that been globally and/or locally locked but pending report generation Allow Global Unlock on Pending Local Lock - to allow users to be set up with the privilege to forcibly unlock a case that is still pending a local lock |
Oracle Argus Safety Administrator's Guide > 2 Access Management > Configuring Users
Oracle Argus Safety Administrator's Guide > 2 System Configuration > Configuring System Management - Common Profile Switches Oracle Argus Safety User's Guide > Global User Management |
| Changes to the Case Locking mechanism in Argus Safety - Changes to global unlocking - Case Form changes | Control globally unlocking a case based on local/global reports pending generation and /or cases pending local lock | Oracle Argus Safety User's Guide > Unlocking a Case |
| Case Form changes - Local Reports Configuration - Local Reporting Rule and Local Reports | Configuring Local Reporting Rules and Local Reports | Oracle Argus Safety Japanese Administrator's Guide > 3 System Configuration > Configuring Local Reports - Local Reporting Rule and Local Reports |
| Triggering Local Reports - Changes to Report Scheduling and Generation Algorithm - Auto Scheduling | Changes to report auto-scheduling for scheduling local reports | Oracle Argus Safety User's Guide > Report Scheduling - Auto-Scheduling |
| Triggering Local Reports - Changes to Report Scheduling Algorithm - Manual Scheduling | Changes to report manual scheduling for scheduling local reports | Oracle Argus Safety User's Guide > Report Scheduling - Manual Scheduling |
| Triggering Local Reports - Changes to Report Generation Algorithm | Changes to report generation for generating local reports | Oracle Argus Safety User's Guide > Triggering Local Reports - Report Generation Algorithm |
| Changes to Expedited Reports and Periodic Reports on DLP | Changes to DLP while generating local expedited and periodic reports | Oracle Argus Safety Japanese User's Guide > Reports |
Process an outlier
When a suspect product with a local license is removed on a follow-up, the case remains a Local PRPT Case (Local Potential Reportable Case) until the corresponding nullification/downgrade local reports is generated.
If a customer wants to change Local Reports configuration data after being in production with this release, it is recommended that customers ensure that cases/reports under processing be completed before changing configuration data to avoid unpredictable results. Note that if a customer changes the Local Reports configuration data mid-way where the reports are mid-way processing (e.g., scheduled), the reports will be determined as local/global based on what type it was at the time when the report was scheduled and will be completed processing that way irrespective of current configuration even if inconsistent with the configuration. Also, note that presence of local reports will determine that the case is a local case.
It is also recommended that customers ensure that cases/reports under processing be completed before up taking the Local Locking feature and (thereby) installing the local lock configuration data (refer to Activate local locking in Argus Safety). Note that if the case was mid-way processing (e.g., case was open in data entry workflow) when customer up took the Local Locking feature, a subsequent case save will determine if the case is local or global.
Activate local locking in Argus Safety
In order to activate the Local Locking feature in Argus Safety, the installer provides the users with an option to install the underlying metadata that enables the local lock feature in the application. A customer, who may not prefer to turn on the local locking feature due to existing business processes that already handle the local processing needs for a company, could choose not to install the metadata and thus not uptake the local lock feature in the application.
A separate database script is provided so that the user can run to turn on the Local lock feature after an upgrade or fresh install. On executing this script, it prompts the user to choose to turn on the local locking feature and uptake the Local Locking seed data.
The application assumes the default value of 1, parses the user input and installs the Local Locking seed data for each of the enterprise specified in the comma separated list.
Enter local reports configuration seed data
A new table is seeded to identify Local Reports for Japan:
-
Country - The seed data is the country id for Japan (from codelist Countries) for this release.
-
Reporting Destination - The script prompts the customer to provide the default value of reporting destination for PMDA.
Prompt - "Please enter Agency Name for the PMDA reporting destination as configured in the "Reporting Destination" codelist. This name will be used to identify Local Reports for all enterprises".
-
Report Form - The seed data is the following Japan reports:

Enter local users seed data
The upgrade installer script prompts the user to choose if all the Argus J users will be updated to have local locking privileges.
Enter local fields seed data
Note that the CMN_FIELDS are always seeded as part of install/upgrade factory data to identify the Local Fields for Japan and will be present irrespective of if the customer chooses local locking feature or not.
In case of Multi-tenancy, the customer input value is used to set the seed values across all enterprises.
This seeding of Local users data is audit logged with the system user.
After install/upgrade, if the customer has turned on the local locking, the icons in the applicable screens start reflecting the local/global lock status of the case.