|
|
| HDR Version 7 HL7 Version 3 Messaging Conformance Specification |
| Contents Previous Next Conformance Examples |
This section contains the following topics:
5.79.1 Introduction 5.79.2 RMIM 5.79.3 Referenced CMETs 5.79.4 Links to Artifacts
The R_Specimen (universal) CMET describes Specimen Material, either manufactured or collected from a patient, including a collection procedure and information that aids in the analysis of the specimen. This CMET also includes information about the container, source, collection procedure, specimen registration, constituent materials, additives, manufacturer and so on. The specimen itself may be a result of a process and can be acted on by any number of processes to handle both the specimen and the container.
The following figure shows the R_Specimen (universal) RMIM:
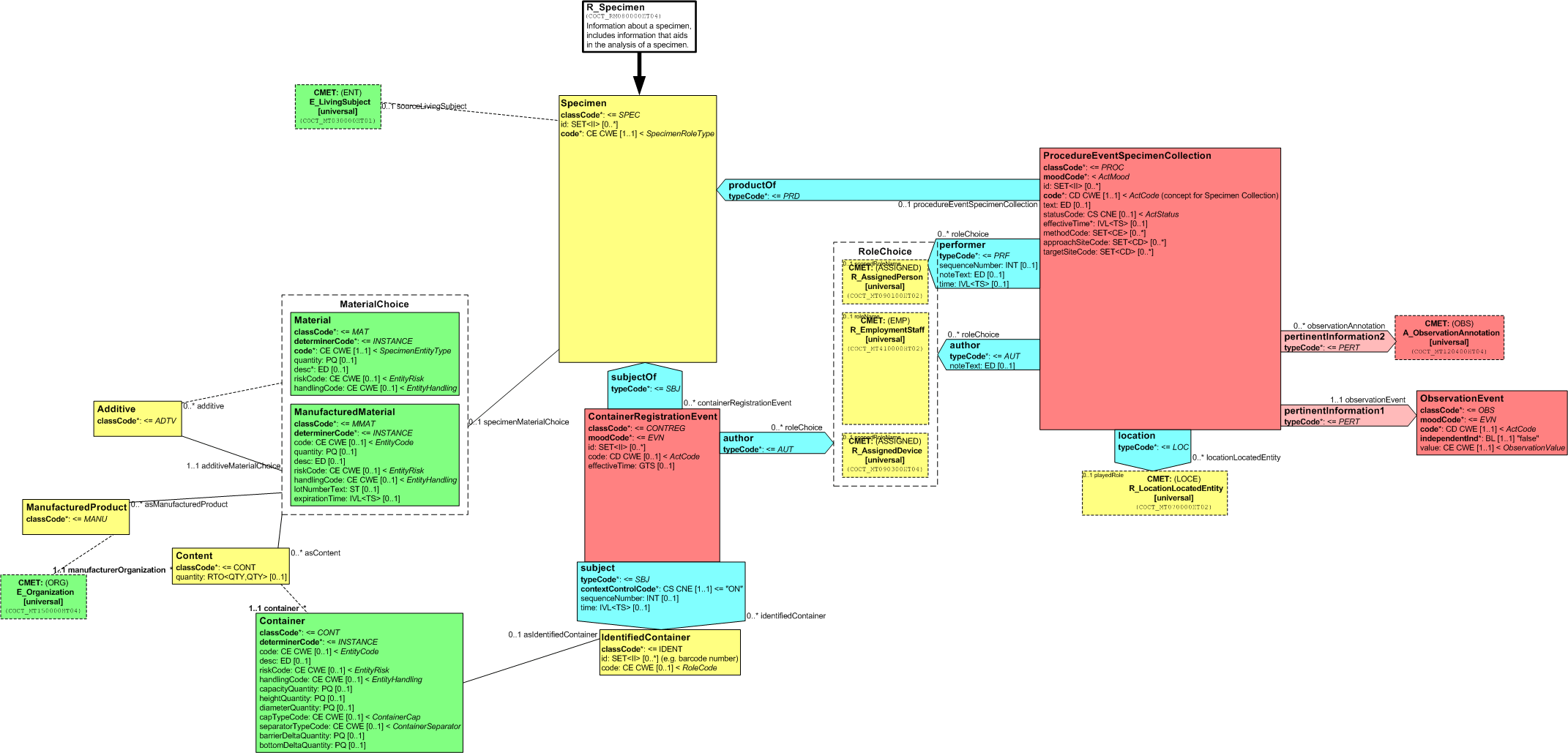
Figure: 5.79.2-1 R_Specimen (universal) RMIM
| Artifacts | Link |
| Visio File | coct_rm080000ht04.vsd |
| Visio XML File | COCT_MT080000HT04.xml |
| Hierarchical Message Description (HMD) | |
| Schema | coct_mt080000ht04.xsd |
| Model Interchange Files (MIF) | coct_mt080000ht04.mif |
| Contents Previous Next Conformance Examples |
|
Copyright © 2018, Oracle. All rights reserved. |
About Oracle Contact Us Legal Notices and Terms for Use Privacy Statement |