A Flexible Study Design Examples
This section contains examples of defining rules in an enhanced DCI Book to support a flexible trial protocol:
For information about flexible studies, see "Designing a Flexible Study" and "Defining an Enhanced DCI Book".
A.1 Basic Examples
Following are examples of how to set up a flexible study in Oracle Clinical by defining DCI Book rules. In the diagrams:
-
Only the setup directly relevant to the example is described. In a real trial, all Intervals would have at least one CPE and all CPEs would have at least one DCI, for example. In addition, each DCI must contain at least one DCM, which must contain at least one Question Group, which in turn contains at least one Question.
-
(U) means unconditional and (C) means conditional. By definition, a conditional DCI or Interval is the target of a rule, and an unconditional DCI or Interval is not.
This section includes the following examples:
A.1.1 Enabling a DCI within a Clinical Planned Event
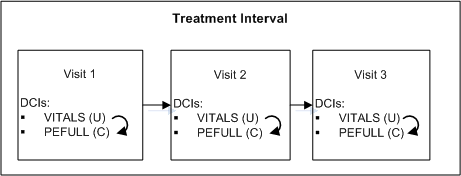
During the treatment phase of a trial, a physical exam is required at each Clinical Planned Event if and only if the patient's vitals have changed since the last Clinical Planned Event.
Figure A-1 Conditional DCI within a Clinical Planned Event
Description of ''Figure A-1 Conditional DCI within a Clinical Planned Event''
To implement this design in Oracle Clinical:
-
Define two DCIs: VITALS and PEFULL and assign them to every CPE in the Treatment Interval.
-
At the end of the VITALS DCI, define Question PE_CHANGE asking "Have there been any changes to vitals since the last visit?" with a Yes/No Discrete Value Group.
-
Define a DCI rule of type "Enable DCI Within CPE" with PE_CHANGE as the trigger Question, Yes as the trigger value, and PEFULL as the target DCI.
At each Clinical Planned Event, the response to the PE_CHANGE Question will determine whether the PEFULL DCI is required for the patient; if Yes, the exam is expected and RDC Onsite displays the corresponding CRF for the patient. If No is entered, it is not expected.
Note:
One rule covers every case where the VITALS and PEFULL DCIs occur in the same CPE.A.1.2 Enabling DCIs Across Clinical Planned Events
A trial has a substudy that requires an extra blood draw at Visits 1, 2, and 3 of the Treatment phase. Not all patients should participate in the substudy.
Figure A-2 Conditional DCI Across Clinical Planned Events
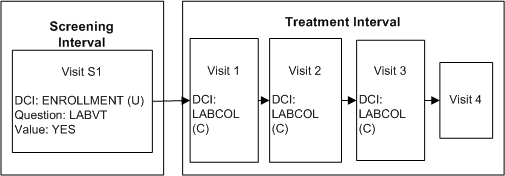
Description of ''Figure A-2 Conditional DCI Across Clinical Planned Events''
To implement this design in Oracle Clinical:
-
Define DCI ENROLLMENT and assign it to Visit S1 in the Screening Interval.
-
Define Question LABVT in DCI ENROLLMENT with a Yes/No Discrete Value Group.
-
Define DCI LABCOL to request the blood draw and record the lab results, and assign it to Visit T1 in the Treatment Interval.
-
Define a DCI rule of type "Enable DCI Across CPEs" with LABVT as the trigger Question, Yes as the trigger value, and LABCOLL as the target DCI.
If a value of Yes is entered for Question LABVT, DCI LABCOLL becomes expected for the patient at every CPE to which it is assigned. RDC Onsite displays the corresponding CRF in visits that are currently expected. If a value of No is entered, DCI LABCOLL is never expected for the patient.
Note:
One rule enables DCI LABCOLL in every CPE to which it is assigned. If you want the same blood test to be performed but without being conditional on the response to Question LABVT, copy the DCI, rename it, and assign the renamed DCI to the appropriate CPEs. If you want it to be conditional upon a different Question response, define another rule. If you want it to be performed on all patients unconditionally, do not define any rule with it as target.A.1.3 Enabling Intervals Based on the Collection of Any Data
All patients in a trial have the same visit schedule expected, going from the screening Interval to the treatment Interval, to the end-of-study Interval.
Figure A-3 Enable Intervals Based on Any Data
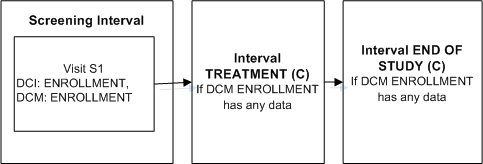
Description of ''Figure A-3 Enable Intervals Based on Any Data''
To implement this design in Oracle Clinical:
-
Define DCI ENROLLMENT that includes DCM ENROLLMENT and assign it to Visit S1 in the Screening Interval.
-
Define Intervals TREATMENT and an END OF STUDY.
-
Define an Interval rule with action "Enable" with DCM ENROLLMENT as the trigger and [Any Data] as the trigger value, and Intervals TREATMENT and END OF STUDY as the target.
As soon as an operator enters any data for a Question in DCM ENROLLMENT and saves, Intervals TREATMENT and END OF STUDY and all their CPEs and unconditional DCIs become expected for the patient. RDC Onsite displays all corresponding expected CRFs and all visits that contain expected CRFs.
Note:
An Interval rule with action "Enable" can make one or more Intervals expected for a patient.A.1.4 Enabling Intervals Based on a Data Value
Patients in a trial must be assigned to one of two treatment arms depending on their response to a Question collected in the Enrollment DCI at the first visit.
Figure A-4 Enable Intervals Based on a Data Value
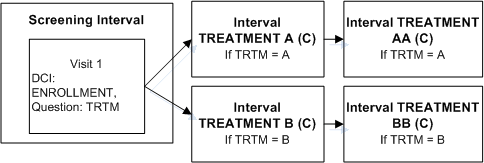
Description of ''Figure A-4 Enable Intervals Based on a Data Value''
To implement this design in Oracle Clinical:
-
Define DCI ENROLLMENT and assign it to Visit S1 in the Screening Interval.
-
Define Question TRTM in DCI ENROLLMENT with a Discrete Value Group that includes values A and B.
-
Define two Intervals, TREATMENT A and TREATMENT B.
-
Define an Interval rule with action "Enable" with TRTM as the trigger Question, A as the trigger value, and Intervals TREATMENT A and TREATMENT AA as the target.
-
Define an Interval rule with action "Enable" with TRTM as the trigger Question, B as the trigger value, and Intervals TREATMENT B TREATMENT BB as the target.
If a value of A is entered for Question TRTM, Interval TREATMENT A and all its CPEs and unconditional DCIs become expected for the patient. If a value of B is entered for Question TRTM, Interval TREATMENT B and all its CPEs and unconditional DCIs become expected for the patient. RDC Onsite displays all corresponding expected CRFs and all visits that contain expected CRFs.
Note:
An Interval rule with action "Enable" can make one or more Intervals expected for a patient.A.1.5 Skipping to an Interval
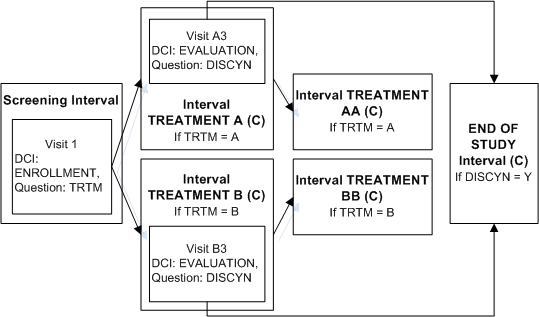
In the same trial, patients in both treatment arms may need to exit the study after the first treatment Interval. An evaluation DCI must be collected ending with a summary Question to collect the decision to discontinue the patient from the study or not.
To implement this design in Oracle Clinical:
-
Define the Screening Interval and Intervals TREATMENT A, TREATMENT AA, TREATMENT B, TREATMENT BB as in the example "Enabling Intervals Based on a Data Value".
-
Define Interval END OF STUDY.
-
Define DCI EVALUATION and assign to Visit A3 in Interval TREATMENT A and Visit B3 in TREATMENT B.
-
Define Question DISCYN in DCI Evaluation with a Yes/No Discrete Value Group assigned.
-
Define an Interval rule with action "Bypass To" with DISCYN as the trigger Question, Yes as the trigger value, and Interval END OF STUDY as the target.
If a value of Yes is entered for Question DISCNY, Interval TREATMENT AA is no longer expected for a patient in TREATMENT A; or TREATMENT BB is no longer expected a patient in Interval TREATMENT B. In both cases Interval END OF STUDY becomes the next expected Interval. RDC Onsite no longer displays either TREATMENT AA or TREATMENT BB but does display all CRFs corresponding to the unconditional DCIs in Interval END OF STUDY and the Visit(s) that contain them.
If a value of No is entered for Question DISCNY, there is no change. The patient completing TREATMENT A proceeds to TREATMENT AA and the patient completing TREATMENT B proceeds to TREATMENT BB. RDC Onsite continues to display all CRFs corresponding to the unconditional DCIs in those Intervals and the Visit(s) that contain them.
Note:
In this trial, you can use any of several methods to ensure that patients who continue to TREATMENT AA or BB also complete the END OF STUDY Interval. For example, define an "Enable" Interval rule with [Any Data] in the first DCM scheduled for the first visit of TREATMENT AA as the trigger and Interval END OF STUDY as the target. Define a similar Interval rule for TREATMENT BB. The diagram then looks like Figure A-6.An Interval can be the target of two rules if one rule has the action "Enable" and the other has the action "Bypass To."
Figure A-6 Enabling and Bypassing To the Same Interval
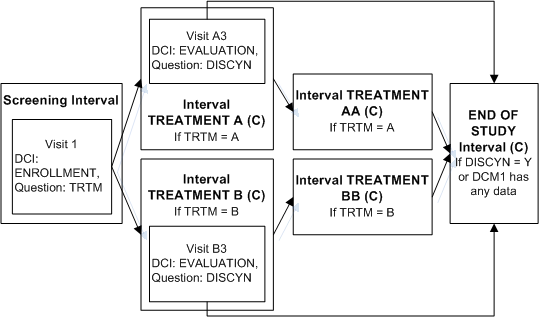
Description of ''Figure A-6 Enabling and Bypassing To the Same Interval''
A.1.6 Enabling the Next Interval
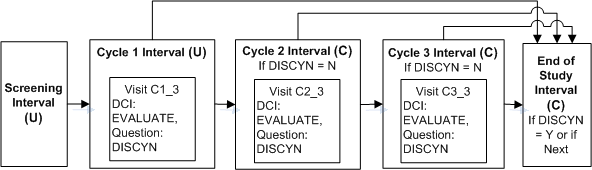
A trial requires a screening Interval followed by three cycle Intervals. The cycle Intervals are exactly the same, but each patient will move to the next cycle only if he or she passes an evaluation in the current cycle and the Discontinue? Question has No entered as a response. When the patient completes all three cycle Intervals or fails the evaluation he or she proceeds to the End of Study Interval.
To implement this design in Oracle Clinical:
-
Define the Intervals SCREENING, END OF STUDY, and Interval CYCLE 1.
-
Define DCI EVALUATION and assign to Visit C3 in Interval CYCLE 1.
-
Define Question DISCYN in DCI EVALUATION with a Yes/No Discrete Value Group assigned.
-
Define Intervals CYCLE 1, CYCLE 2, and CYCLE 3. Add their CPEs to the DCI Book, add DCIs to CYCLE 1, then copy those pages to the CPEs in the other cycle Intervals; see "Copying Pages for a CPE".
-
Define an Interval rule with action "Enable" with DISCYN as the trigger Question, No as the trigger value, and [Next Interval] as the target.
-
Define an Interval rule with action "Bypass To: with DISCYN as the trigger Question, Yes as the trigger value, and Interval END OF STUDY as the target.
If a value of No is entered for Question DISCNY, the next CYCLEx Interval becomes expected for the patient. If a value of Yes is entered for DISCYN, the END OF STUDY Interval becomes expected for the patient. Until a value is entered for DISCYN, neither the next CYCLE Interval nor the END OF STUDY Interval is displayed in RDC Onsite.
Note:
You must be careful to define more CYCLE Intervals than you will need. If not, every patient in the last defined CYCLE Interval proceeds to END OF STUDY because it is the Next Interval and therefore the target of both the Enable and the Bypass To rules.If you need to add cycles after entering data, you must define addition Intervals in the study schedule, then set the Book to Provisional and add DCI Book Pages to the Intervals' CPEs, and set the Book's status back to Active.
A.2 Complex Examples
The following examples show how multiple rules interact:
A.2.1 Defining Multiple Paths Through a Trial
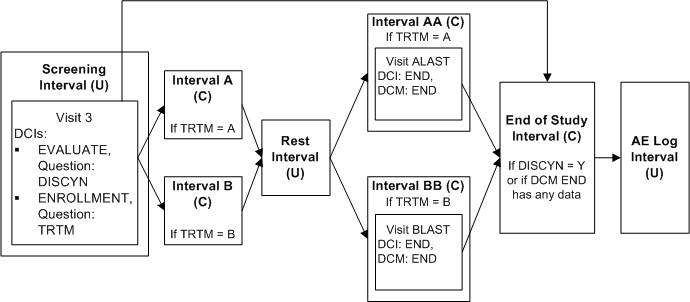
This trial includes two treatment paths:
-
Screening Interval, Interval A, Rest Interval, Interval AA, End of Study Interval, AE Log Interval
-
Screening Interval, Interval B, Rest Interval, Interval BB, End of Study Interval, AE Log Interval
Both paths share the Screening, Rest, End of Study Intervals, and AE Log. In addition, if certain criteria are not met the path goes directly from the Screening Interval to the End of Study Interval.
To implement this design in Oracle Clinical:
-
Define Intervals Screening, A, B, Rest, AA, BB, End of Study and AE Log in this order in the study schedule.
-
Define DCI EVALUATE with Question DISCYN with a DVG with values Yes and No, and assign it to Visit 3 in Screening Interval.
-
Define DCI ENROLLMENT with Question TRTM with a DVG with values A and B and assign it also to Visit 3 in Screening Interval.
-
Define DCI END with DCM END and assign it to Visit ALAST in Interval AA and Visit BLAST in Interval BB.
-
Define Interval rule 1 with action "Enable" as follows:
-
Trigger: DCI ENROLLMENT, Question TRTM, value A, CPE Visit 3
-
Target: Interval A, Interval AA
-
-
Define Interval rule 2 with action "Enable" as follows:
-
Trigger: DCI ENROLLMENT, Question TRTM, value B, CPE Visit 3
-
Target: Interval B, Interval BB
-
-
Define Interval rule 3 with action "Enable" as follows:
-
Trigger: DCI END, DCM END [Any Data], (Question null, value null), CPE ALAST, BLAST
-
Target: Interval End of Study
-
RDC Onsite displays the Screening, Rest, and AE Log Intervals and all their unconditional DCIs and the CPEs that contain them, for each patient assigned to the DCI Book, before any data is entered. If you prefer not to display the Rest and AE Log Intervals until they are the next expected Interval, you can define a rule with a trigger in the preceding Interval and a target of [Next Interval].
If the response to DISCYN is N and the response to TRTM is A, all Intervals in path A become expected for the patient and RDC Onsite displays all the unconditional DCIs in Intervals A and AA and the Visit(s) that contain them. If the response DISCYN is N and the response to TRTM is B, the system does the same for path B. In both cases, the End of Study Interval becomes expected for the patient when DCM END has any saved data.
However, if the response to DISCYN is Y, the patient proceeds immediately to the End of Study Interval even a value of A or B is entered for TRTM. None of the other Intervals is either expected or displayed in RDC Onsite.
Notes:
You can define an Enable Interval rule triggered in Interval AA and BB even though a Bypass rule exists that bypasses Intervals AA and BB when triggered.The Rest and AE Log Intervals are automatically expected for patients on both paths because they are defined in that order in the study schedule and are not the target of any rule.
A.2.2 Enabling DCIs in a Conditional Interval
This trial has two treatment paths. In path A only, male patients are required to have an extra blood draw. Both male and female patients are assigned to both treatment paths.
Figure A-9 Conditional DCIs in a Conditional Interval
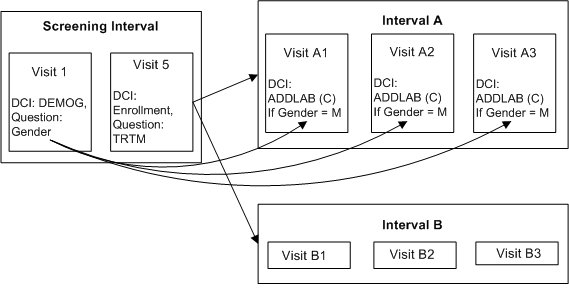
Description of ''Figure A-9 Conditional DCIs in a Conditional Interval''
To implement this design in Oracle Clinical:
-
Define Intervals Screening, A and B.
-
Define DCI DEMOG with Question GENDER with a DVG with values M and F, and assign it to Visit 1 in the Screening Interval.
-
Define DCI ENROLLMENT with Question TRTM with a DVG with values A and B, and assign it to Visit 5 of the Screening Interval.
-
Define DCI ADDLAB and assign it to Visits A1, A2, and A3 in Interval A.
-
Define Interval Rule 1 with action "Enable" as follows:
-
Trigger: DCI ENROLLMENT, Question TRTM, value A, CPE Visit 5
-
Target: Interval A
-
-
Define Interval Rule 2 with action "Enable" as follows:
-
Trigger: DCI ENROLLMENT, Question TRTM, value B, CPE Visit 5
-
Target: Interval B
-
-
Define DCI Rule 3 of type "Enable Across CPEs" as follows:
-
Trigger: DCI DEMOG, Question Gender, value M, CPE Visit 1
-
Target: DCI ADDLAB
-
At Visit 1, the response to the trigger Question GENDER is entered. However, no DCIs become expected for the patient even if the trigger response (M) is entered because the patient is not yet assigned to either Interval A or B. After the response to Question TRTM is entered for Visit 5 either Interval A or B becomes expected for the patient. If TRTM's value is A and GENDER's value is M, then DCI ADDLAB becomes expected in Visits A1, A2, and A3 in Interval A. Only then does RDC Onsite display both Interval A with all its unconditional DCIs and the CPEs that contain them, and DCI ADDLAB for male patients.
Note:
Target DCIs can be contained in multiple CPEs in an Interval that is made expected by an Interval rule triggered by a DCI in a CPE later than the CPE that contains the DCI rule trigger.A.2.3 Defining Multiple Conditional Pretreatment Intervals and DCIs
This trial has a single treatment path that includes a Screening, Treatment, and End of Study Interval. In addition, all male patients must complete a PROSTATE DCI during the Screening Interval and one of two additional Intervals (SPECIAL A or SPECIAL B) based on a response in the PROSTATE DCI. Patients in SPECIAL A must also complete a XHIST DCI if they have a history of heart disease.
Figure A-10 Multiple Conditional Pretreatment Intervals and DCIs
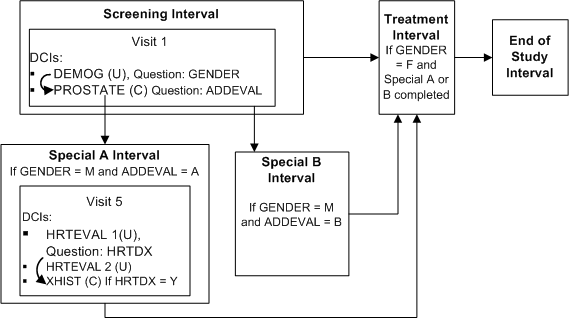
Description of ''Figure A-10 Multiple Conditional Pretreatment Intervals and DCIs''
To implement this design in Oracle Clinical:
-
Define Intervals Screening, Special A, Special B, Treatment, and End of Study in that order in the study schedule
-
Define DCI DEMOG DCI with Question GENDER with a DVG with values M and F, and assign it to Visit 1 in the Screening Interval
-
Define DCI PROSTATE with Question ADDEVAL with a DVG with values A and B, and assign it to Visit 1 in the Screening Interval
-
Define DCI HRTEVAL1 with Question HRTDX with a DVG with values Y and N, and assign it to Visit 5 in Interval Special A
-
Define DCI XHIST and assign it to Visit 5 in Interval Special A
-
Define Interval Rule 1 with action "Enable" as follows:
-
Trigger: DCI DEMOG, Question GENDER, value F, CPE Visit 1
-
Target: Treatment Interval
-
-
Define DCI Rule 2 of type "Enable Within CPE" as follows:
-
Trigger: DCI DEMOG, Question Gender, value M, CPE Visit 1
-
Target: DCI: PROSTATE
-
-
Define DCI Rule 3 of type "Enable Within CPE" as follows:
-
Trigger: DCI HRTEVAL 1, Question HRTDX, value Y, CPE Visit 5
-
Target: DCI: XHIST
-
When a value of M is entered for Question Gender at Visit 1, the PROSTATE DCI becomes expected for the patient and must be collected at the same visit. If the value of Question ADDEVAL in the PROSTATE DCI is A, Interval Special A becomes expected for the patient. If the value of ADDEVAL is B, Interval Special B becomes expected. Patients in Interval Special A must complete the HRTEVAL DCI, and if their response to Question HRTDX is Y, they must also complete DCI XHIST.
Male patients proceed to the Treatment Interval after completing either Interval Special A or Interval Special B because it is defined next in the study schedule. Female patients proceed to the Treatment Interval immediately after completing the Screening Interval. The Screening and End of Study Intervals are expected and displayed in RDC Onsite for all patients from the beginning of the trial.
RDC Onsite displays Intervals Special A and Special B and the conditional DCIs within them only when they become expected for each patient.