13 Configuring Settings for Reports
This chapter includes the following topics:
-
Section 13.1, "Defining Settings for the Patient Data Report"
-
Section 13.2, "About the File Name for the Deleted CRFs Report"
-
Section 13.4, "Printing Left Default Prompts for Repeating Response Fields"
13.1 Defining Settings for the Patient Data Report
Using Oracle Clinical, you can configure several settings that control what information is included in a Patient Data Report. You can control:
-
Bookmarks in the PDF report file
-
Whether to include the approval and verification information for each CRF, the audit history for fields not displayed in the CRF, and the overflow text for hidden protected repeating defaults
-
Whether the pages in the table of contents and the cover page are counted when determining the starting page number for the CRFs
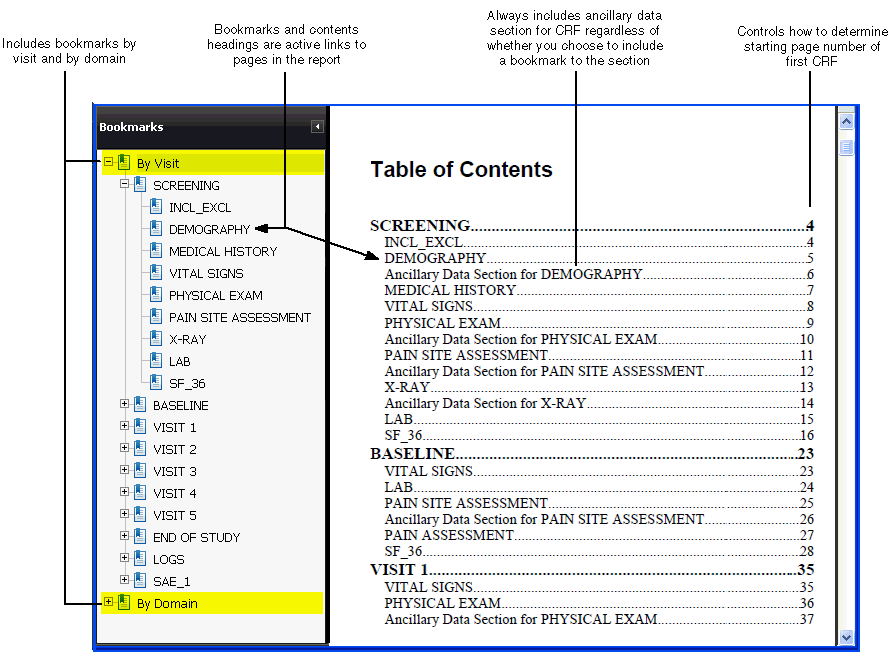
The system automatically generates a Table of Contents (TOC) for every report. When you open and view the report, you can click a heading on the Table of Contents pages to go to the selected CRF. The headings, like the bookmarks, are active links to the CRFs.
13.1.1 Defining the Configuration Settings for the Patient Data Report
In Oracle Clinical, you can define the configuration settings for the Patient Data Report at the database level or at the study level:
-
At the database level, the Patient Data Report settings define the default values when a new study is created. For each setting, you can choose to enforce the default value across all studies in the database or allow modification at the study level. You define the default values in the DCI Form Local Database Settings form.
-
At the study level, the Patient Data Report settings control the output of the Patient Data Report. You can define all settings at the study level except the PDR Bookmark Data Domain setting.
13.1.1.1 Defining Report Settings at the Database Level
To define the Patient Data Report settings at the database level:
-
Open Oracle Clinical.
-
Navigate to Admin and select DCI Form Local Database Settings.
-
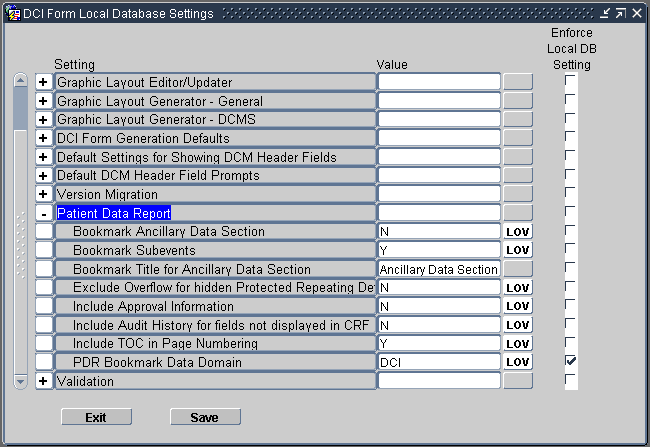
Expand the Patient Data Report node.
Description of the illustration ''pdr_custom_set.gif''
-
Change the value of the settings you want. See Table 13-1 for a description of each setting.
-
Select the Enforce Local DB Setting check box to always use this value as the default value.
If not selected, a user who has privileges to the DCI Form Study Database Settings form at the study level can override the default values.
Note that the Enforce Local DB Setting check box is always selected for the PDR Bookmark Data Domain setting because you cannot change its value at the study level.
-
Save your changes.
13.1.1.2 Defining Report Settings at the Study Level
To define the Patient Data Report settings at the study level:
-
Open Oracle Clinical.
-
Navigate to Design and select DCI Form Local Study Settings.
-
Expand the Patient Data Report node.
-
Deselect the Inherit From Local DB Setting check box for any value you want to change.
-
Change the value of the settings you want. See Table 13-1 for a description of each setting.
-
Save your changes.
13.1.2 About the Patient Data Report Settings
Table 13-1 describes the settings that you can use to control the output of the Patient Data Report.
Table 13-1 Settings for the Patient Data Report
| Setting | Description |
|---|---|
|
Bookmark Ancillary Data Section |
Specifies whether to create bookmarks for the Ancillary Data section associated with a CRF.
Note that the Patient Data Report always includes the Ancillary Data section for a CRF regardless of whether you choose to bookmark the section. If you choose to bookmark the section, you can specify the title of the bookmark by entering text in the Bookmark Title for Ancillary Data Section setting. |
|
Bookmark Subevents |
Specifies whether to create bookmarks for the subevents of a visit.
|
|
Bookmark Title for Ancillary Data Section |
Specifies the title to use for the bookmarks to the Ancillary Data sections of the CRFs. To create the bookmark, RDC appends your specified title to the name of any CRF that has an associated Ancillary Data section. Bookmark Format: Bookmark-title for CRF-name Example: Ancillary Data Section for DEMOGRAPHY This setting is valid only if the Bookmark Ancillary Data Section setting is set to Y. The default value is Ancillary Data Section. |
|
Exclude Overflow for Hidden Protected Repeating Default |
Specifies whether the report excludes the overflow text for all repeating default questions that are hidden.
Note: If the CRF response field for a protected repeating default is less than one character long and this value is set to Y, the Ancillary Data section does not list the values for the response field. By using this combination, you can hide certain fields in a CRF. |
|
Include Approval Information |
Specifies whether to include two types of approval information for each CRF: approval history and for approved documents, the approver and date and time of approval. This setting has effect only if you select the Include Audit History check box when generating the Patient Data Report from the Reports page in RDC or if you specify the -aud parameter when generating the report from the command line. If set to Y, the Ancillary Data section for each approved CRF includes the following information:
|
|
Include Verification Information |
Specifies whether to include the verification status and history for each CRF.
This setting has effect only if you select the Include Audit History check box and user has one or more of the Verify privileges (BROWSE_VERIFY, BROWSE_SDVPLAN, UPDATE_SDVPLAN, or VERIFY) when generating the Patient Data Report from the Reports page in RDC Onsite or if you specify the -aud parameter when generating the report from the command line. |
|
Include Audit History for Fields Not Displayed in CRF |
Specifies whether to include the audit history for CRF fields that are not displayed in the CRF.
One example of how CRF data is collected but not displayed on the CRF is the Blank Flag icon. In certain circumstances, RDC users may need to mark a CRF as intentionally blank; for example, if a patient fails to appear for a scheduled visit. Users can click the Blank Flag icon to mark a CRF as blank. RDC keeps an audit history of all changes to a CRF. This setting has effect only if you select the Include Audit History check box when generating the Patient Data Report from the Reports page in RDC or if you specify the -aud parameter when generating the report from the command line. |
|
Include TOC in Page Numbering |
Determines how RDC calculates the starting page number for the CRFs listed in the Table of Contents (TOC).
|
|
PDR Bookmark Data Domain |
Specifies whether to consider the DCI or the DCM as the data domain for the purpose of creating the bookmarks. For the report, the system generates two sets of bookmarks: By Visit and By Domain. See Figure 13-1 for an example of these bookmarks.
You can configure this setting only at the database level. Note that the Enforce Local DB Setting check box is always selected for the PDR Bookmark Data Domain setting because you cannot change its value at the study. |
13.2 About the File Name for the Deleted CRFs Report
The name of the PDF output file for the Deleted CRFs Report is constructed based on the Short Values defined in the Oracle Clinical local reference codelist PDR_FILE_NAMING, which you can modify in limited ways:
-
You can change the order of the 3 components of the name.
-
You can mark any or all components as inactive.
In addition, the string DEL and the job ID are appended at the end of the resulting file name.
The file name format is as follows:
StudyName_StudySiteName_PatientID_DEL_JobID.pdf
Where:
-
StudyName can be up to 15 characters.
-
StudySiteName can be up to 10 characters.
-
PatientID can be up to 10 characters.
-
JobID can be up to 10 characters.
For example, pdfrun_ss002_10012_DEL_23546.pdf.
The batch jobs table can accommodate an output file name of up to 70 characters, which includes the reports directory path concatenated to the output file name. If your study name, study site name, and patient ID all use the maximum length allowed by Oracle Clinical, the file name itself will use 52 characters: StudyName(15)_StudySiteName(10)_PatientID(10)_JobID(10).pdf, leaving 18 characters for the directory path. If the 70-character limit is exceeded, the file name will be of the shortened form: oJobID.pdf
Also, if all components in the Oracle Clinical local reference codelist PDR_FILE_NAMING are inactive, the file name will be of the shortened form: oJobID.pdf is used.
Lastly, if the study or study site names include any spaces, those spaces are replaced in the report file name with underscores (_).
13.3 Customizing Bookmark Labels
The RDC Client package contains PL*SQL procedures that you can modify to change the labels for the Domain and Visit bookmarks:
-
DCI bookmark label
-
DCM bookmark label
-
Visit bookmark label
For details on changing these labels, see Chapter 8, "Working with the RDC Client Package."
13.4 Printing Left Default Prompts for Repeating Response Fields
When you setup and define DCM questions in Oracle Clinical, you can define repeating default values for repeating question groups.
To print left repeat default prompts for each repeating response in a CRF:
-
Enter the default response prompt when you define the DCM question in a DCM.
-
Select Left as the prompt position when defining the DCM question group.
Once defined in Oracle Clinical, RDC automatically includes the default repeat prompts for all defined responses to print (and display) in the DCM question group in the CRF:
-
When you print a Blank Casebook Report
-
When you open a new blank CRF in RDC
-