9 Approving CRFs
In Oracle Clinical Remote Data Capture Onsite (RDC Onsite), an approval is the equivalent of an electronic signature. Generally, the site investigator approves CRFs. You can approve CRFs only if you have approval privileges.
You approve CRFs, one at a time, from the Data Entry window. Alternatively, your sponsor can configure RDC Onsite to make a group approval option available. Techniques for finding the CRFs requiring approval vary slightly, depending upon whether or not you have a Source Data Verification (SDV) plan in effect for the study site. If you do have a published SDV plan in place, then partial SDV is likely in effect, whereby not all CRFs require SDV. You can view the SDV requirement for CRFs only if you have the BROWSE_VERIFY or higher privilege. See Using Source Data Verification.
This chapter includes the following topics:
Finding CRFs for Approval
To review patient CRFs and approve the data, you can:
-
Use a customized Activities link on the Home page
-
Select patients on the Home page and navigate to the Review CRFs page
-
Select patients on the Home page and navigate to the Patient Casebooks page
Using a Customized Activities Link on the Home Page
By default, the Activities section on the Home page includes a Review non-blank CRFs ready for initial approval link. This link has built-in search criteria that automatically finds only those CRFs that are ready for approval, and then displays those CRFs on the Review CRFs page.
Your sponsor can customize the Activities links. They can choose to create additional links, define different search criteria for links, rename links, or make some links unavailable. The links available to you depends on how your sponsor customized the RDC Onsite application.
To use the default Activities link to find CRFs for approval:
-
Select the Review non-blank CRFs ready for initial approval link in the Activities section on the Home page.
The Review CRFs page lists all the patients and their CRFs. If the Approved column is blank, then the CRF is not approved. If Partial Source Data Verification is implemented for your site, the CRFs page lists only those CRFs that require source data verification according to the SDV Plan in place.
-
Expand the Search pane to view the criteria in effect as a result of selecting the activity.
-
Click Reset to specify different search criteria, if needed.
-
Click Search to refresh the page according to your new criteria.
Selecting Patients and Navigating to the Review CRFs Page
You can select specific patients for review on the Home page, and then navigate to the Review CRFs page to view the CRFs ready for approval.
To navigate to the Review CRFs page and view CRFs ready for approval:
-
Open the Home page and expand the Search pane.
-
Specify patient search criteria. For example, you can specify a patient range, patients assigned to a particular casebook (study protocol), or patients with no open discrepancies.
-
Click Search to refresh the patient list according to your criteria.
-
Select one or more patients for review from the Patients list. In order to do that, you can click one row and then use the Ctrl + A keys to select all the patients displayed.
Alternatively, you can click on the table header for the patient icon to select all cells.
Note:
You can use combinations of keys including Ctrl on all supported devices except for iPad. -
Click the Action menu and then select Review CRFs from the list.
The Review CRFs page opens, displaying all entered CRFs for the selected patients.
-
Expand the Search pane to specify CRF-level criteria, such as verification status, entry status, or discrepancy status.
-
Click Search to refresh the CRF list after selecting additional criteria.
To reset your patient selection entirely and specify a different search, click Reset.
Selecting Patients and Navigating to the Patient Casebooks Page
You can also select specific patients for review on the Home page, and then navigate to the Patient Casebooks page, instead of the Review CRFs page, to view the CRFs ready for approval. Navigating to the Patient Casebooks page lets you review CRFs in a casebook spreadsheet format instead of a list format.
To navigate to the Patient Casebooks page and view CRFs ready for approval:
-
Open the Home page and expand the Search pane.
-
Specify patient search criteria. For example, you can specify a patient range, patients assigned to a particular casebook (study protocol), or patients with no open discrepancies.
-
Click Search to refresh the patient list according to your criteria.
-
Select one or more patients for review from the Patients list. In order to do that, you can click one row and then use the Ctrl + A keys to select all the patients displayed.
Alternatively, you can click on the table header for the patient icon to select all the cells.
Note:
You can use combinations of keys including Ctrl on all supported devices except for iPad. -
Click the Action. menu and then select Multi-Patient View from the list.
-
The Patient Casebooks page opens, displaying all entered and expected CRFs for the selected patients in spreadsheet format. Each row of icons represents data for a single patient. See "Using the Patient Casebooks Page" for information on navigating in the Patient Casebooks page.
-
Expand the Search pane to specify CRF-level criteria, such as verification status, entry status, or discrepancy status.
-
Click Search to refresh the CRF list after selecting additional criteria.
To reset your patient selection entirely and specify a different search, click Reset.
Note:
When you specify CRF-level criteria, the empty CRF icons are not displayed. Ordinarily, these icons represent data that is expected according to the patient's casebook definition.However, when CRF-level search criteria are in effect, cells where data has not been entered are blank. If patients within the current set do not have data for a particular CRF, the column is not displayed. This feature allows for a more compressed view of patient CRFs.
Finding CRFs Requiring Re-Approval
Data can be updated even after a CRF has been approved. When this happens, the CRF automatically acquires an approval status of Awaiting Re-Approval.
To find CRFs that require re-approval:
-
Follow one of the methods described in "Finding CRFs for Approval" to find CRFs ready for approval.
-
Expand the Search pane on either the Review CRFs page or the Patient Casebooks page.
-
Click the Approval field and then select Awaiting Re-Approval from the list.
-
Click Search to refresh the display.
-
Continue to "Reviewing Changes since Latest Approval".
Note:
CRFs with an Awaiting Re-Approval status are not retrieved when the Approval field is set to Not Approved.Reviewing CRFs from the Data Entry Window
After identifying CRFs that require approval, you can review them to ensure that they meet the requirements for approval. Your review of CRFs can include:
Reviewing Data
To review entered data for errors:
-
Open a CRF in a Data Entry window.
-
Review data in the CRF fields.
-
Change data as required.
-
Save the CRF, if you change data.
Tip:
Click the Next CRF link to navigate through all CRFs for a patient visit without having to return to the Patient Casebooks page or the Review page.Reviewing Discrepancies
As an investigator, you may need to review discrepancies before you approve a CRF. See Chapter 4, "Managing Discrepancies" for more information.
Reviewing Approval History
Before you approve a CRF, RDC Onsite lets you view the CRF's approval history, if any. A CRF that has never been approved does not have an approval history.
To view the approval history of a CRF:
-
Open the CRF in a Data Entry window.
-
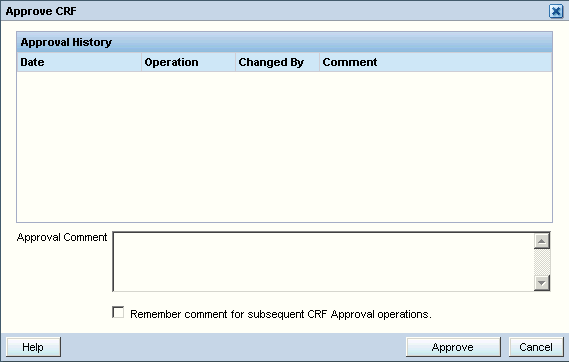
Click the Approve icon in the toolbar. The Approve CRFs dialog box displays the following information about the approval history, if any, for the CRF.
Date — Displays the date and time of the entry in the Approval History table.
Operation — Displays the action that caused a change in approval status: Approve or Undo Approval.
Changed By — Displays the full name of the user who changed the approval status.
Comment — Displays additional information about the Approve or Undo Approval action.
-
Close the Approve CRFs dialog box.
Reviewing Changes since Latest Approval
The data in a CRF can undergo change even after the CRF has been approved. When this happens, RDC Onsite automatically changes the status of the CRF to Awaiting Re-Approval.
You should always review the changes in a CRF before you approve it again.
To review the changes to a CRF since the latest approval:
-
Search for a CRF that needs to be re-approved:
-
Open the Patient Casebooks page or the Review CRFs page.
-
Expand the Search pane.
-
Click the Approval field and then select Awaiting Re-Approval from the list.
-
Click Search to execute the search and refresh the display.
Note:
If you set the Approval field to Not Approved, the search does not retrieve CRFs with an Awaiting Re-Approval status.
-
-
Open a CRF in a Data Entry window.
-
Click the Highlight field in the toolbar, and select Changed since last approved from the list. Fields updated since the last approval are highlighted in blue. Note that fields whose only update is an update to the investigator comment are highlighted.
-
Right-click a field highlighted in blue, and select View Audit History. The Audit History pane opens at the bottom of the Data Entry window.
-
Review the history of changes for the selected field.
-
Continue to review the changes to other fields in the CRF since the last approval.
Finding Non-highlighted Changes in a CRF
When you select Changed since last approved in the Highlight field in the Data Entry window, RDC Onsite does not highlight fields that have undergone the following changes:
-
If updates were restricted to discrepancies, RDC Onsite does not highlight the associated fields. To locate such cases:
-
-
Click the Approve icon in the Data Entry window toolbar to establish the date of the latest approval.
-
Review the Discrepancy History to identify any updates since the last approval.
-
-
If the layout of a CRF was modified, the CRF status reverts to Awaiting Re-Approval even without any data updates.
Follow your sponsor's instructions for re-approving CRFs with non-highlighted changes.
Reviewing Audit History
As an investigator, you need to review changes to a CRF, especially the changes made to a CRF that was already approved. The Audit History pane provides comprehensive information on the changes to each field in a CRF.
To view the history of the changes to a field:
-
Open a CRF in a Data Entry window.
-
Right-click a field and then select View Audit History. The Audit History pane opens at the bottom of the Data Entry window.
-
Review the history of changes for the selected field.
Note:
The Audit History pane does not include those changes made to discrepancies or investigator comments. You can, however, view the Discrepancy History and the Investigator Comment History for information on those updates.Approving CRFs from the Data Entry Window
After identifying CRFs ready for approval as described in "Finding CRFs for Approval", and reviewing CRFs as described in "Reviewing CRFs from the Data Entry Window", you can approve an individual CRF from the Data Entry window. You can use a similar process to undo an approval as well as to redo the approval of a CRF.
To approve a CRF:
-
Open a CRF in a Data Entry window.
-
Click the Approve icon in the toolbar to open the Approve CRF dialog box.
Description of the illustration ''approvecrf.gif''
The Approve CRFs dialog box includes the approval history of the CRF, if any.
The approval actions available in the Approve CRF dialog box depends on the current status of the CRF. In addition, the Approve button does not appear if the CRF has not been saved, if the CRF was saved as incomplete, or if you do not have approval privileges for the CRF.
Based on the current status of the CRF, you can:
-
Click Approve to approve the CRF.
-
Click UnApprove to undo the approval if the CRF was already approved.
-
Click Re-Approve to redo the approval of a CRF. A CRF needs to be re-approved if data is updated or added since the last approval.
-
-
Save your changes.
See "Reviewing Approval History" if you want to review the approval changes to the CRF.
Redoing CRF Approval
RDC Onsite assigns Awaiting Re-Approval status to a CRF if data is updated after the CRF was approved.
To redo the approval of a CRF:
-
Search for CRFs that need to be re-approved, as described in "Finding CRFs Requiring Re-Approval".
-
Review changes in the CRF after approval, as described in "Reviewing Changes since Latest Approval".
-
Review the audit history of changes in the CRF, as described in "Reviewing Audit History", if you want.
-
Redo the approval of an individual CRF, as described in "Approving CRFs from the Data Entry Window".
-
Redo the approval of a group of CRFs if you need to, as described in "Approving Multiple CRFs".
Approving Multiple CRFs
You can approve multiple CRFs simultaneously from the Patient Casebooks page or the Review CRFs page. The group approval option is available only if you have approve privileges, and if your sponsor has configured RDC Onsite to allow it.
To approve multiple CRFs:
-
Open the Patient Casebooks page or the Review CRFs page.
-
From the Multi-patient casebook page, select one or more patients whose CRFs you need to approve. Click the Action menu and select Approve from the list.
-
From the Single patient casebook page, select one or more visits whose CRFs you need to approve. Click the Action menu and select Approve from the list.
-
From the Review CRFs page, select one or more CRFs to approve. Click the Action menu and select Approve from the list.
Note:
If the Approve option does not appear in the list, either you do not have appropriate privileges, or your sponsor has not configured RDC Onsite for group approval.The Group Approval dialog box opens and displays the following information:
-
The number of CRFs selected
-
The number of selected CRFs with open discrepancies
-
The number of unverified CRFs
-
The number of patients you selected (if you started with a patient selection from the Multiple Patients View of the Patient Casebooks page.)
-
-
Enter a comment in the Approval Comment field, if you want.
-
In the Exclude set of options, clear the Exclude CRFs with discrepancies check box if you want to approve such CRFs.
Other options you have listed in the Exclude area:
-
Non-migrated CRFs entered from Oracle Clinical or RDC Classic
-
Batch Loaded CRFs
These two options are not checked by default.
-
-
Choose by clicking the checkbox corresponding to one of the options listed in the Include section, if this is the case:
-
Verified CRFs only
-
CRFs for this visit only (Otherwise, all visits for the selected patient(s)).
-
-
Click Continue to proceed with the group approval.
RDC Onsite approves all the selected CRFs. A dialog box reports when the approval is complete, and displays the total number of CRFs that were approved.
-
Click Ok or on the background page to close window.
RDC Onsite automatically refreshes the page and updates the approval status of the affected CRF icons.
Note:
The number of CRFs approved may be less than the number of CRFs selected for any of the following reasons:-
Any of the optional checkboxes is selected, depending on the settings from Oracle Clinical.
-
The CRF is already approved.
-
The CRF has a database lock (another user or a validation procedure is updating the CRF).
-
The CRF has an Oracle Clinical lock.
-
The CRF is entered from another site.
-
The CRF is at status Received or Entry Started.
Undoing Approval of Multiple CRFs
If you unintentionally approve a group of CRFs, you can reverse the action with the Undo Group Approval option. You can undo approval of multiple CRFs simultaneously from the Patient Casebooks page or the Review CRFs page.
The Undo Group Approval option is available only if you have the appropriate privileges, and your sponsor has configured RDC Onsite to allow it.
To undo the approval of a group of CRFs:
-
Open the Patient Casebooks page or the Review CRFs page.
-
From the Multi-patient casebook page, select one or more patients whose CRFs you need to unapprove. Click the Action menu and select UnApprove from the list.
-
From the Single patient casebook page, select one or more patients whose CRFs you need to unapprove. Click the Action menu and select UnApprove from the list.
-
From the Review CRFs page, select one or more CRFs for which you need to undo the approval. Click the Action menu and select UnApprove from the list.
Note:
If the UnApprove option does not appear in the list, either you do not have appropriate privileges, or your sponsor has not configured RDC Onsite for the undo group approval.The UnApprove CRFs dialog box opens and displays the following information:
-
The number of CRFs selected
-
The number of patients you selected (if you started with a patient selection from the Multiple Patients View of the Patient Casebooks page.)
You have the option to include CRFs for this visit only, by checking the check box in the Include section, under the comment text box.
-
-
Enter the user name and password that you specify when you log in to the RDC Onsite application.
-
Enter a comment, if you want.
-
Click Continue to proceed with undoing the group approval.
RDC Onsite undoes the approval of the selected CRFs. A dialog box reports when the unapprove operation is complete, and displays the total number of CRFs that were unapproved.
-
Click Ok to close the dialog box.
RDC Onsite automatically refreshes the page and updates the approval status of the affected CRF icons.
About the Approve CRFs Dialog Box
RDC Onsite prompts you to enter your username and password before you approve a CRF or undo the approval of a CRF.
The following rules apply:
-
For the first CRF approval action, enter the same user name and password that you use to log in to RDC Onsite.
-
For subsequent approvals, you only need to enter your password. However, if your session times out in between, you need to enter both your user name and password. Contact your Help Desk for information on the timeout period.
-
RDC Onsite allows three attempts to enter the correct password. After three failed attempts, RDC Onsite displays a warning that the Data Entry window will close and your session will end. Click OK to close the window and end your session.