11 Viewing Summary Reports
Oracle Clinical Remote Data Capture Onsite (RDC Onsite) summarizes information about studies, sites, and patients into one-page reports. These reports, which you can view and print, let you track your progress throughout the study or clinical trial.
This chapter includes the following topics:
Viewing a Study and Site Summary Report
The one-page Study and Site Summary Report includes up-to-date information on the total number of patients and CRFs in the entire study and at the selected site. The metrics include counts for patients and CRFs at various statuses in the study.
To view and print the Study and Site Summary Report:
-
Open the Home, Patient Casebooks, or Reports page.
You can change the study and site before viewing the summary report from the Home or Patient Casebooks page. The Reports page has no option to change the study and site.
-
Click the Study and Site Summary link located on the top right of the page.
Description of the illustration ''reports_stdy_site_lk.gif''
RDC Onsite opens a new window and displays up-to-date metrics for the current study and site. Figure 11-1 shows a sample Study and Site Summary Report.
-
To print the Study and Site Summary Report, click Print. Use a Landscape orientation to format the report properly on the printed page.
-
To close the window and return to the previous page, click Close.
-
Figure 11-1 Study and Site Summary Report
Description of ''Figure 11-1 Study and Site Summary Report''
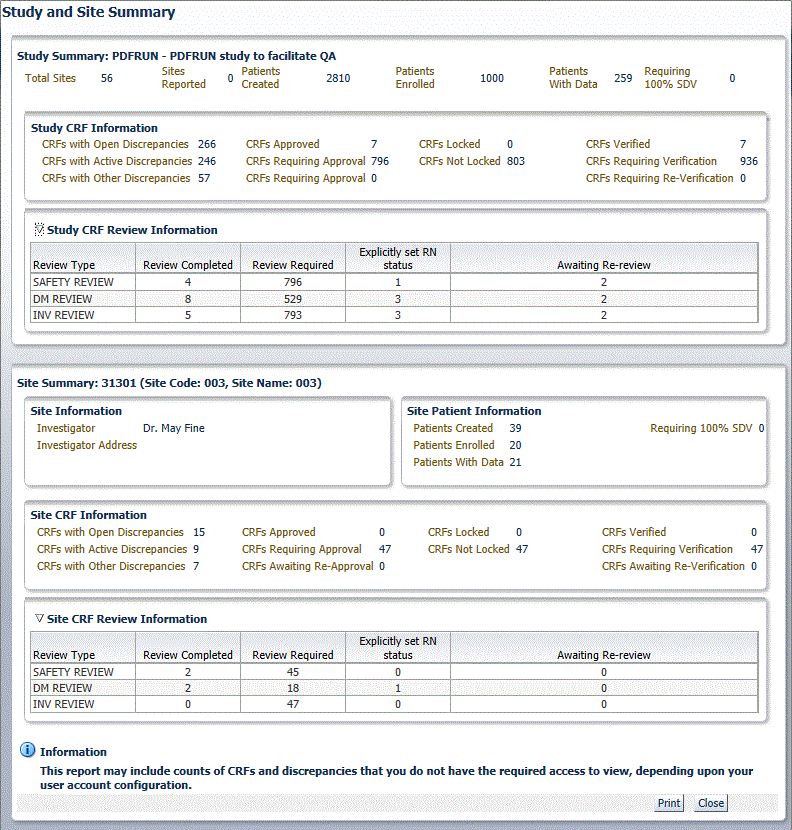
The Study Summary section lists the overall metrics for the currently selected study. You can view metrics only for the sites to which you have privileges.
-
Study Information — Lists the total number of sites in the study, the number of sites in the study for which you have access to view the information, and the number of patients created, patients enrolled, and patients with data entered for the study.
-
Study CRF Information — Lists the total number of planned and unplanned CRFs for the study, and the total number of CRFs across the study with various discrepancy, verification, lock, approval statuses, and custom review statuses. Verification and custom review metrics appear only if you have the appropriate privileges.
The Site Summary section lists the overall metrics for the current site.
-
Site Information — Lists the address of the site, and the name and address of the site investigator.
-
Site Patient Information — Lists the number of patients created, patients enrolled, patients with data entered for the site and patients requiring 100% Source Data Verification (SDV).
-
Site CRF Information — Lists the total number of planned and unplanned CRFs for the site, and the sum totals of CRFs with various verification, lock, discrepancy, and approval statuses.
About the Counts in the Study and Site Summary
-
The Study and Site Summary may include counts of CRFs and discrepancies that you do not have the required access to view, depending upon your user account configuration.
-
The counts include the CRFs and discrepancies that you do not have the required access to view, if any, depending upon your user account configuration.
-
The counts for Planned CRFs include all conditional CRFs that have become expected.
-
The CRF counts in the Site Summary section are based on the CRF site assignment. If data entry takes place in two sites for the same patient, RDC Onsite apportions the CRF counts between the two sites.
Viewing a Patient Summary Report
In addition to viewing a summary report for the current study and site, you can view and print the latest overall metrics for a single patient.
To view or print a summary report for only one patient:
-
Open either the Home page or the Patient Casebooks page.
-
Search for the patient.
-
Click the patient icon to display a summary report for the selected patient.
Description of the illustration ''reports_pat_sum_icon.gif''
RDC Onsite opens a new window and displays up-to-date metrics for the current patient. Figure 11-2 shows a sample Patient Summary Report.
-
To print the Patient Summary Report, click Print. Use a Landscape orientation to format the report properly on the printed page.
-
To close the window and return to the previous page, click Close.
-
About the Patient Summary Report
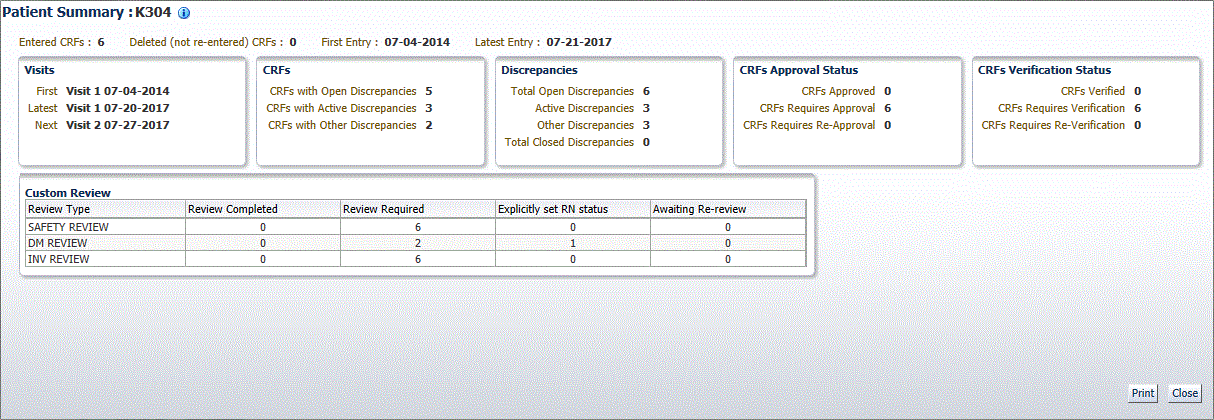
As shown in Figure 11-2, the Patient Summary Report includes the following information for the selected patient:
-
Patient Information — Lists the patient number and, if available, lists the birth date, gender, and age of the patient, and the date of the latest update to the CRF. If there is an active SDV plan in place, and the patient is selected for the verification, the title displays 100% SDV required.
-
Discrepancies — Lists the number of active discrepancies, other discrepancies, and closed discrepancies for the patient. In addition, lists the total number of discrepancies (active, other, and closed).
-
CRFs — Lists the number of CRFs with active discrepancies, with other discrepancies, and with no open discrepancies. In addition, lists the total number of CRFs with discrepancies, the date of the first entry (earliest) to a CRF, and the date of the latest entry.
-
CRF Custom Review Status - for each custom review type for which you have privileges, lists the number of CRFs with the following statuses: review completed, review required, explicitly set RN status, and awaiting re-review.
-
CRFs Verification Status — Appears if you have the appropriate privileges. Lists the number of CRFs with the following verification statuses: verified, requires verification, and requires re-verification.
-
CRFs Approval Status — Lists the number of CRFs with the following approval statuses: approved, requires approval, and requires re-approval.
-
Links — Lists up to three custom links that open other Web pages and provide additional information on the patient. These links are available only if your sponsor customized the Patient Summary Report to include the links. For example, your sponsor may include a link to the Adverse Events for the patient.
About the Counts in the Patient Summary Report
-
The counts include the CRFs and discrepancies that you do not have the required access to view, if any, depending upon your user account configuration.
-
The counts include for all CRFs associated with the selected patient, regardless of the patient's current site assignment.