| Oracle® Clinical Remote Data Capture Onsite User's Guide Release 4.6.2 Part Number E18822-01 |
|
|
View PDF |
| Oracle® Clinical Remote Data Capture Onsite User's Guide Release 4.6.2 Part Number E18822-01 |
|
|
View PDF |
You can generate two types of reports in RDC Onsite: the Patient Data Report and the Blank Casebook Report. See Chapter 11, "Generating CRF Reports" for details.
This appendix includes the following topics:
A Patient Data Report (PDR) includes all the CRFs entered against a patient. Optionally, the report can include discrepancy and audit information as well. You can use this report for electronic submissions, and as a printout for off-line discussion and review.
A Patient Data Report contains a list of all data entered for the patient including data entered for the patient at another site, if the user running the report has access to that site.
A Patient Data Report has certain standard components, and some components specific to certain types of reports, or to certain conditions, such as long data values being present.
In addition to the cover page, a Patient Data Report can include the following sections:
A CRF data section
Ancillary Data pages if the CRF has any response overflow, audit, discrepancy, or investigator comment information
A Deleted CRFs section
An Appendix listing CRFs not included in the report
Every Patient Data Report has a cover page that lists the following basic information:
Title — The title of the report. The title uses the following format:
CRF Report for Study study-name.
Report generated by — The full name of the user who initiated the report.
On — The date and time the report was generated.
Report Parameters — A list of the selected parameters for the report, including:
The patient ID for which the report was run.
The search criteria in effect on the Home or Casebooks page when the report was run.
Legends — An explanation of the time stamps and the superscripts shown in the report.
Figure B-1 shows a sample cover page for a Patient Data Report.
The CRFs in a Patient Data Report may include one or more fields marked with a superscript number. Superscript numbers are assigned sequentially in a CRF, based on the order of the fields.
The superscript indicates that the field is associated with one of the following conditions:
Overflow — The entered text is too large for the field.
Investigator Comment — The field has an investigator's comment associated with the response.
Discrepancies — The field is associated with one or more discrepancies. A section discrepancy is associated with the first field in the section.
Audit History — The field has undergone modification since originally entered.
If a superscript is on the CRF, an Ancillary Data page follows immediately after the last page of the CRF.
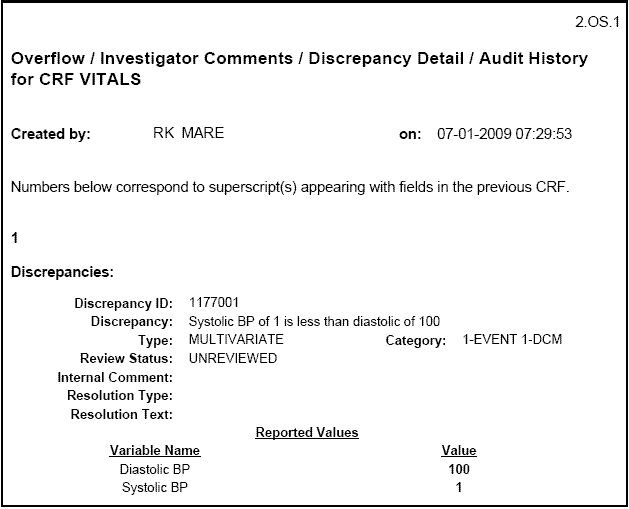
An Ancillary Data page lists each superscript number found in a CRF and outputs any additional information belonging to the corresponding field. The additional information may include any overflow text, investigator comments, discrepancy details, and audit history associated with the field. Figure B-2 shows a sample Ancillary Data page.
The Ancillary Data page includes the user name (ID) of the individual who created the CRF, and the date and time the CRF was created. For example:
Created by: RK MARE on: 07-01-2009 07:29:53
Note that the creation date and time marks when the CRF was first saved, regardless of whether it was saved as complete or incomplete.
If configured to show Approval information, the Ancillary Data page also includes the name of the individual who approved the CRF, and the date and time the CRF was approved. For example:
Approved by: JOHN SMITH on: 08-21-2009 11:15:22
Figure B-2 Sample Ancillary Data Page in a Patient Data Report
A Patient Data Report automatically includes any overflow text and investigator comments associated with a field. The report includes discrepancy and audit information only if you:
Select the Include Discrepancy Details and the Include Audit History check boxes on the Reports page.
Generate the report from the Reports page.
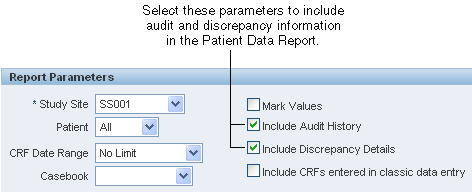
If you specify to include an audit history, the Patient Data Report includes an audit history for all fields, even for those fields (responses) that have never been updated.
The Ancillary Data page includes the user name (ID) of the individual who created the CRF, and the date and time the CRF was created. Note that the creation date and time marks when the CRF was first saved, regardless of whether it was saved as complete or incomplete.
When combined with the field-level audit history, the report provides a complete history for each field with the following exceptions:
For responses initially saved as null while the CRF is in status Pass1 Started (or Entry Started), the time at which a response for the field changed from null to not null is not available. Once the CRF is saved complete, the field-level audit history includes an entry for the change from null to not null.
If optional CRF header fields (like Comment) are not entered during the initial data entry, the time at which a response for the field changes from null to not null is not available.
If a CRF is marked blank with the initial save, and responses are subsequently entered, the time at which you entered a response after you cleared the Is Blank check box is not available.
However, the history for the Is Blank check box itself indicates the time at which you cleared the check box. You can assume that you entered at least one response, if not all responses, at that time. Furthermore, if you save the CRF as complete at this time, the Patient Data Report includes subsequent changes of other fields from null to not null values as part of the audit histories for those fields.
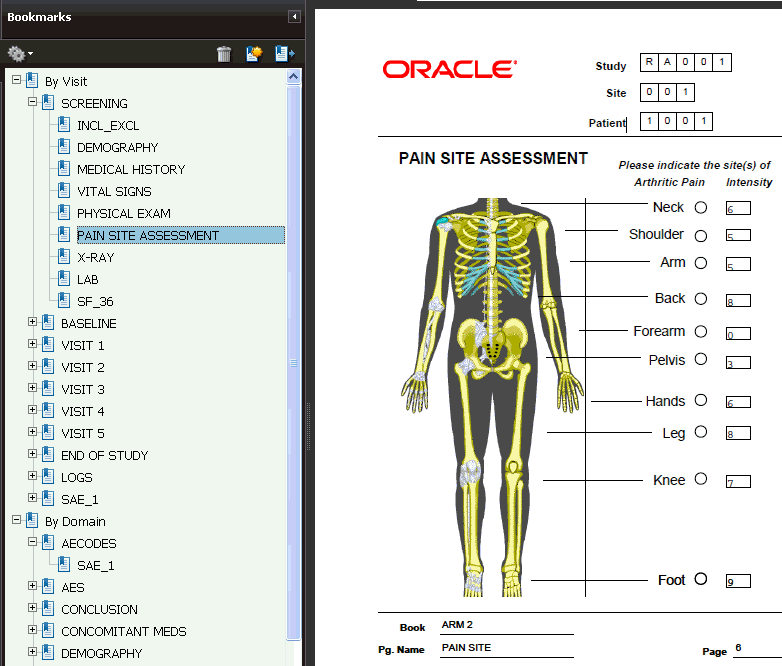
Every Patient Data Report includes a table of contents with bookmarks to the various topics and pages in the report. See Figure B-3. You can click the bookmarks to access pages in the report.
The report also includes two sets of bookmarks:
The first set is organized by Visit and within the visit, the Data Domain.
The second set is organized by Data Domain and within a Data Domain, by Visit.
For example, if an Adverse Events CRF is entered for Visit 1 and Visit 2, the report has two AE pages in the report. A bookmark would exist under Visit 1, AE as well as under AE, Visit 1. You can click either of the bookmarks to open the first AE page entered for Visit 1.
You can navigate to the cover page of the report by clicking on the By Visit bookmark or the By Domain bookmark.
Figure B-3 Bookmarks for Reviewing the Patient Data Report
The Patient Data Report may include the following appendices:
A listing of all deleted CRFs, if an audit history is included in the report.
A listing of all CRFs not included for the particular Patient Data Report. A CRF is not included if a layout does not exist for it.
RDC Onsite numbers CRFs according to visits. Within a visit, RDC Onsite numbers the CRFs according to their assigned casebook.
If you add an unplanned page in the casebook, RDC Onsite assigns it the page number of the last expected CRF for the particular visit, appended with U, as well as a unique number for the unplanned page for that visit. For example, if Visit 2 includes pages 3 through 10, the second unplanned CRF entered for Visit 2 receives the number 10.U.2.
In a Patient Data Report, extended text fields output as follows:
On the CRF page, each extended text field displays the ATTACHMENT prefix and the truncated response text.
The extended text field does not use a superscript in the CRF to indicate an overflow of text. Instead, the ATTACHMENT prefix indicates that the field is an extended text field.
For each extended text field in the CRF that has data, the Patient Data Report includes a separate Extended Text Ancillary Data page that displays the full content of the field. The Extended Text Ancillary Data page:
Contains one extended text field per page. If a CRF has more than one extended text field, then each field has a separate Extended Text Ancillary Data page.
Labels the page with the name of the CRF and the name of the extended text field.
Includes page numbers in the upper-right corner.
Note:
Data from an extended text field does not display in the Overflow section of the primary Ancillary Data pages.Optionally, the Patient Data Report can include the audit history for an extended text field.
To include the audit history, select the Include Audit History parameter when you generate the Patient Data Report. If you specify to include an audit history, the Patient Data Report includes an audit history for all fields.
The superscript indicates that an audit history is associated with the field.
The audit history prints on the Ancillary Data pages along with the audit history for standard fields. In this section, the audit history for an extended text field includes only the ATTACHMENT prefix and the truncated response text.
In addition, the Ancillary Data pages include a new section in which the audit history for an extended text field shows the complete extended text from one update to the next update.
To avoid duplicating large amounts of text, the audit history does not include the Changed To field. If there is one audit history entry, the Changed To value prints in the current extended text. If there are multiple audit history entries, the Changed To value is in the previous entry.
The Blank Casebook Report consists of a blank copy of all the planned CRFs in the casebook. Generating and printing a blank casebook is useful for sites still doing paper data entry.
Note:
When you generate a Blank Casebook Report for a flexible study, the report may be quite large because it includes all CRFS defined in the casebook even though for a given patient not all CRFs and visits will be expected.Each generated Blank Casebook Report has a cover page that lists the following basic information about the report:
Title — The title of the report. The title uses the following format:
CRF Report for Study study-name.
Casebook — The name of the casebook designated for the report.
Report generated by — The full name of the user who initiated the report.
On — The date and time the report was generated.
Legends — An explanation of the time stamps and the superscripts shown in the report.
If you specified a patient number when you generated the report, the cover page also includes the patient number (ID) for which the report was run.
Most fields in the Blank Casebook Report are empty so you can hand write patient responses into the blank form. However, RDC Onsite automatically fills in the following fields when generating the report:
Study Name
Visit Name
Visit Number
CRF Name
Short Name
In addition, RDC Onsite displays the Patient ID, Site ID, and Investigator ID on the Blank Casebook Report.