| Oracle® Healthcare Data Model Reference 11g Release 2 (11.2) Part Number E18026-02 |
|
|
View PDF |
| Oracle® Healthcare Data Model Reference 11g Release 2 (11.2) Part Number E18026-02 |
|
|
View PDF |
This chapter provides Oracle Healthcare Data Model sample reports.
This chapter includes the following sections:
Note:
The reports and dashboards shown in the examples in this chapter and delivered with Oracle Healthcare Data Model are provided only for demonstration purposes. These sample reports and dashboards are not supported by Oracle.Provides cardiac sample reports.
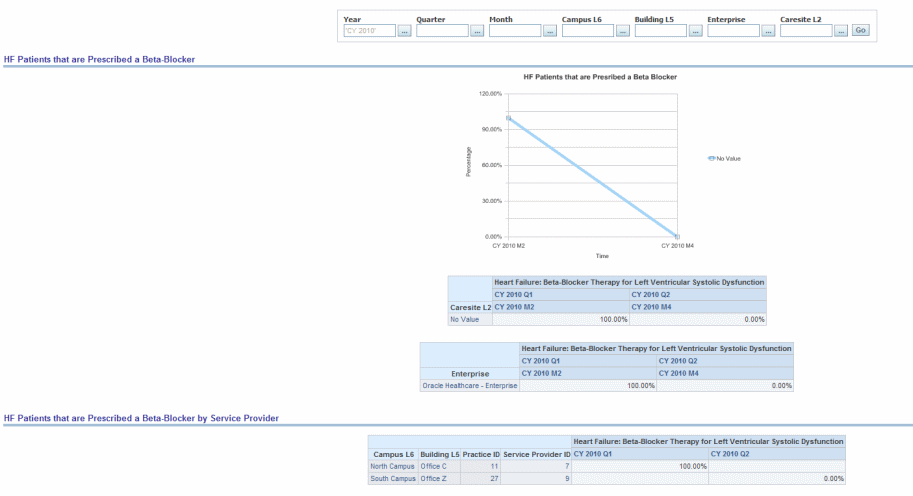
Percentage of patients aged 18 years and older with a diagnosis of heart failure who also have LVSD (LVEF < 40%) and who were prescribed beta-blocker therapy.
Report dimensions are:
Year (Time)
Quarter (Time)
Month (Time)
Campus (Facility)
Building (Facility)
Caresite (Facility)
Enterprise (Organization)
Figure 13-1 Cardiac: PQRI-Heart Failure: Heart Failure Patients Prescribed a Beta-Blocker
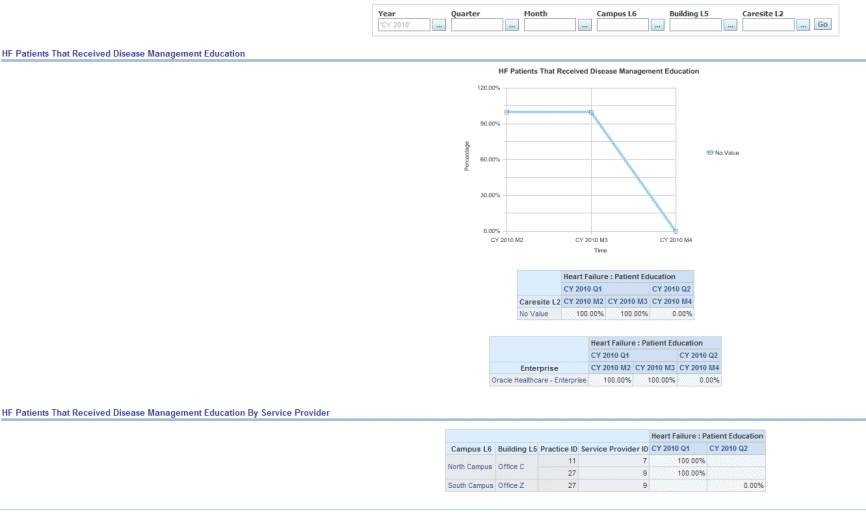
Percentage of patients aged 18 years and older with a diagnosis of heart failure who were provided with patient education on disease management and health behavior changes during one or more visit(s) within 12 months.
Report dimensions are:
Year (Time)
Quarter (Time)
Month (Time)
Campus (Facility)
Building (Facility)
Caresite (Facility)
Enterprise (Organization)
Figure 13-2 Cardiac: PQRI Heart Failure: Heart Failure Patients Received Disease Management Education
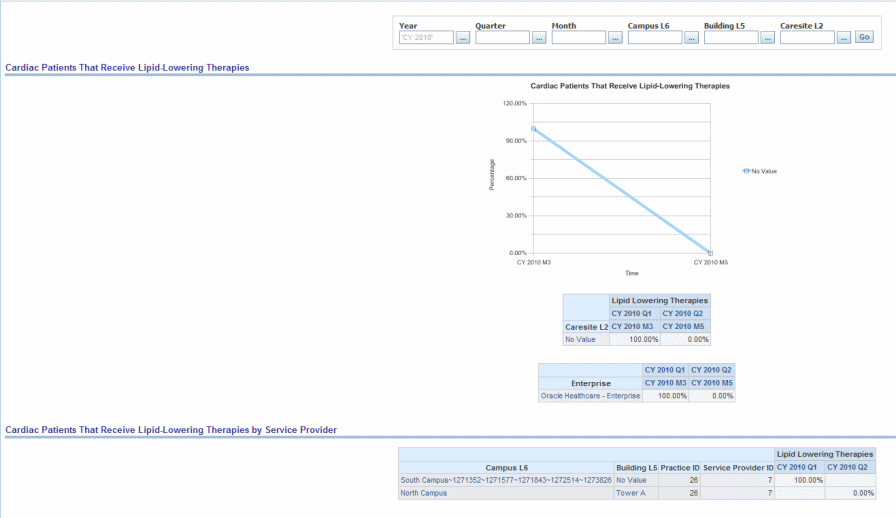
Percent of patients with LDL > 100 who received lipid-lowering therapies or percent of patients who received lipid lowering therapies.
Report dimensions are:
Year (Time)
Quarter (Time)
Month (Time)
Campus (Facility)
Building (Facility)
Caresite (Facility)
Enterprise (Organization)
Figure 13-3 Cardiac: AHA- Cardiac: Cardiac Patients That Receive Lipid-Lowering Therapies
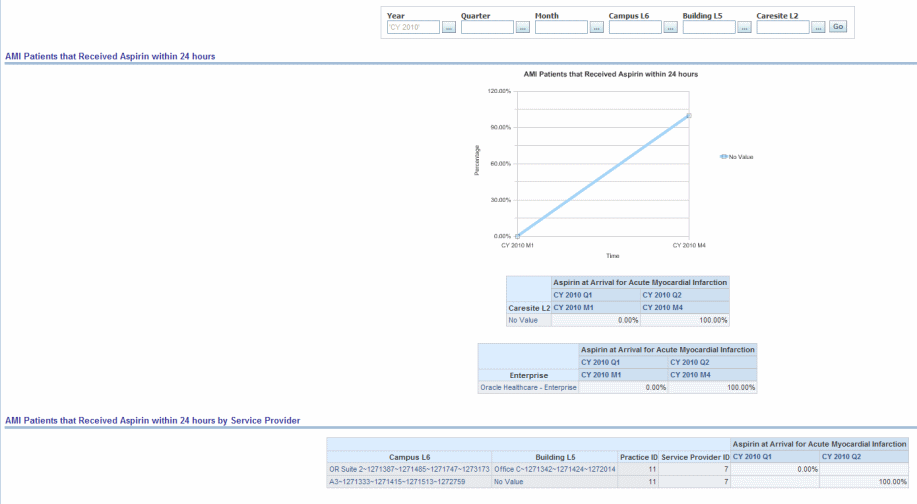
Percentage of patients, regardless of age, with an emergency department discharge diagnosis of Acute Myocardial Infarction (AMI) who had documentation of receiving aspirin within 24 hours before emergency department arrival or during emergency department stay.
Report dimensions are:
Year (Time)
Quarter (Time)
Month (Time)
Campus (Facility)
Building (Facility)
Caresite (Facility)
Enterprise (Organization)
Figure 13-4 Cardiac: PQRI- Cardiac AMI Patients That Received Aspirin Within 24 Hours
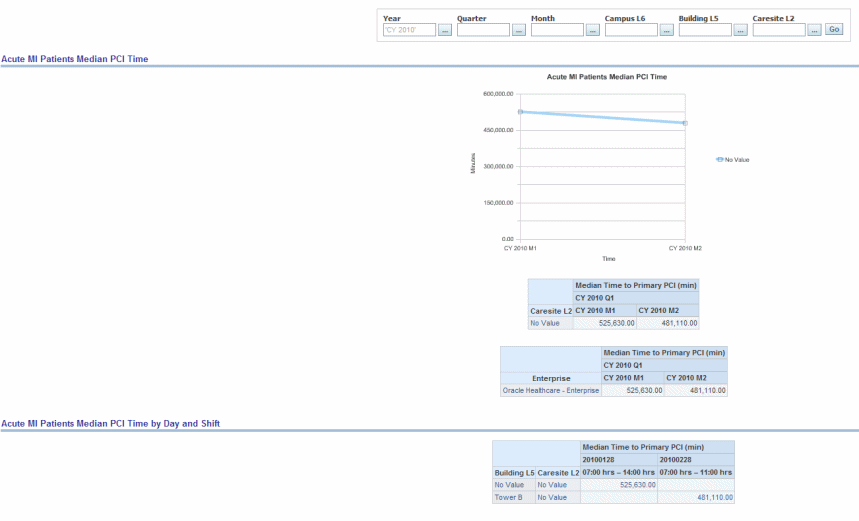
Median time from hospital arrival to primary percutaneous coronary intervention (PCI) in Acute Myocardial Infarction (AMI) patients with ST-segment elevation or left bundle branch block (LBBB) on the electrocardiogram (ECG) performed closest to hospital arrival time.
Report dimensions are:
Year (Time)
Quarter (Time)
Month (Time)
Campus (Facility)
Building (Facility)
Caresite (Facility)
Enterprise (Organization)
Figure 13-5 Cardiac: CMS/JCAHO Cardiac Acute MI Patients Median PCI Time
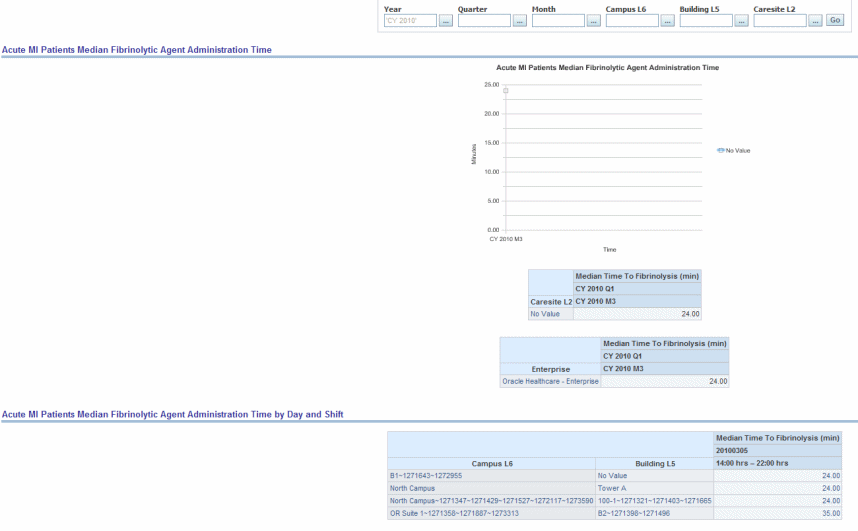
Median time from arrival to administration of fibrinolytic therapy in Acute Myocardial Infarction (AMI) patients with ST-segment elevation or left bundle branch block (LBBB) on the electrocardiogram (ECG) performed closest to hospital arrival time.
Report dimensions are:
Year (Time)
Quarter (Time)
Month (Time)
Campus (Facility)
Building (Facility)
Caresite (Facility)
Enterprise (Organization)
Figure 13-6 Cardiac: CMS/JCAHO Cardiac: Acute MI Patients Median Fibrinolytic Agent Administration Time
Provides diabetes sample reports.
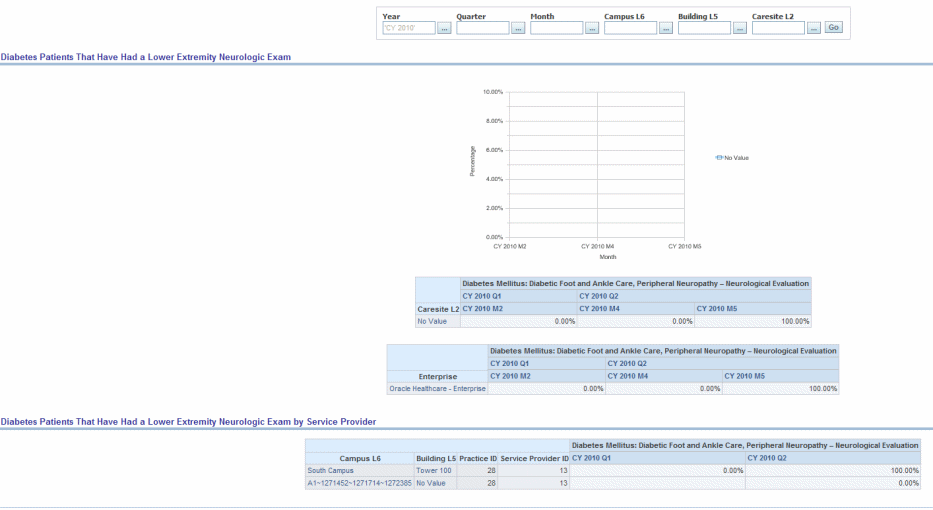
Description: Percentage of patients aged 18 years and older with a diagnosis of diabetes mellitus who had a neurological examination of their lower extremities within 12 months.
Report dimensions:
Year (Time)
Quarter (Time)
Month (Time)
Campus (Facility)
Building (Facility)
Caresite (Facility)
Figure 13-7 PQRI- Diabetes Report: Diabetic Patients That Have Had a Lower Extremity Neurologic Exam
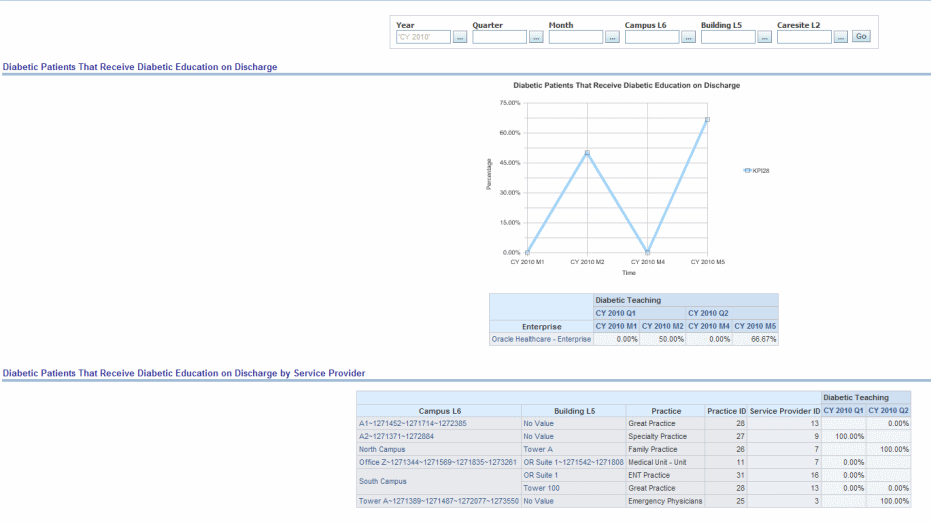
Description: Diabetic Teaching: Percent of diabetic patients or newly diagnosed diabetics receiving diabetes teaching at discharge.
Report dimensions are:
Year (Time)
Quarter (Time)
Month (Time)
Campus (Facility)
Building (Facility)
Caresite (Facility)
Figure 13-8 AHA-Diabetes Report: Diabetic Patients That Receive Diabetic Education on Discharge
Provides general medicine sample reports.
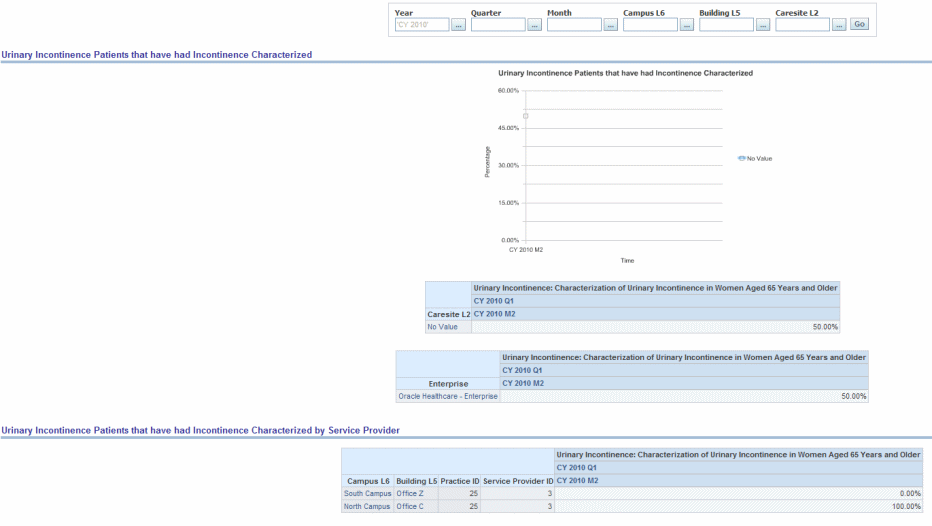
Percentage of female patients aged 65 years and older with a diagnosis of urinary incontinence whose urinary incontinence was characterized at least once within 12 months.
Report dimensions are:
Year (Time)
Quarter (Time)
Month (Time)
Campus (Facility)
Building (Facility)
Caresite (Facility)
Figure 13-9 General Practice PQRI General: Urinary Incontinence Patients had Incontinence Characterized
Percentage of women aged 40 through 69 years who had a mammogram to screen for breast cancer within 24 months. Report is similar to that shown in Figure 13-9.
Report dimensions are:
Year (Time)
Quarter (Time)
Month (Time)
Campus (Facility)
Building (Facility)
Caresite (Facility)
Adults Queried about Tobacco Use
Percentage of patients aged 18 years or older who were queried about tobacco use one or more times within 24 months. Report is similar to that shown in Figure 13-9.
Report dimensions are:
Year (Time)
Quarter (Time)
Month (Time)
Campus (Facility)
Building (Facility)
Caresite (Facility)
This report provides prediction for customer churn probability based on predicts churn SVM probability measures, for different customers. Report is similar to that shown in Figure 13-9.
Report dimensions are:
Year (Time)
Quarter (Time)
Month (Time)
Campus (Facility)
Building (Facility)
Caresite (Facility)
Provides pediatrics sample report.
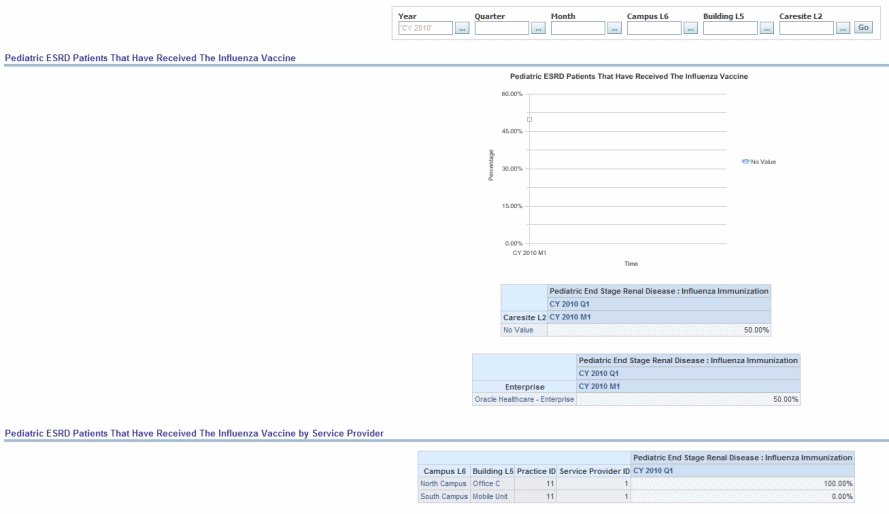
Percentage of patients aged 6 months through 17 years with a diagnosis of ESRD and receiving dialysis seen for a visit between November 1 and February 15 who have documented administration of influenza immunization or patient reported receipt of an influenza immunization from another provider.
Report dimensions are:
Year (Time)
Quarter (Time)
Month (Time)
Campus (Facility)
Building (Facility)
Caresite (Facility)
Figure 13-10 Pediatrics ESRD Patients that Have Received the Influenza Vaccine
Provides psychiatric sample reports.
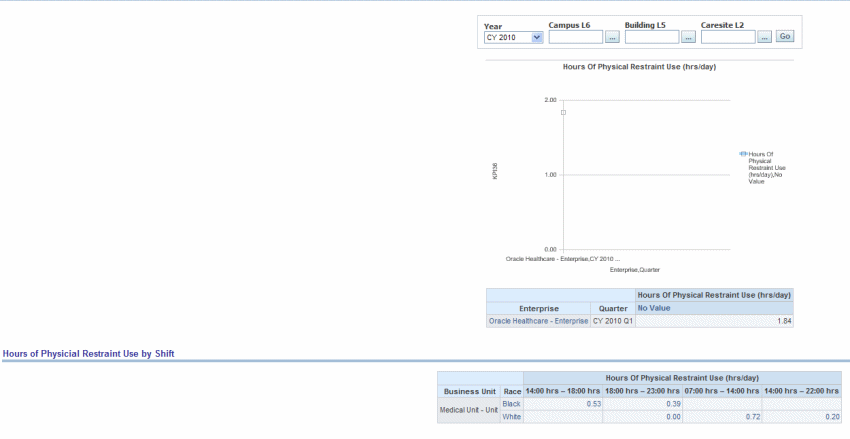
The total numbers of hours that all patients admitted to a hospital-based inpatient psychiatric setting were maintained in physical restraint.
Report dimensions are:
Year (Time)
Quarter (Time)
Month (Time)
Campus (Facility)
Building (Facility)
Caresite (Facility)
Figure 13-11 Psychiatric: Hospital Based Inpatient Psychiatric Services Hours of Physical Restraint Use
The total numbers of hours that all patients admitted to a hospital-based inpatient psychiatric setting were here held in seclusion.
Report dimensions are:
Year (Time)
Quarter (Time)
Month (Time)
Campus (Facility)
Building (Facility)
Caresite (Facility)
Figure 13-12 Psychiatric: Hospital Based Inpatient Psychiatric Services Hours of Seclusion Us
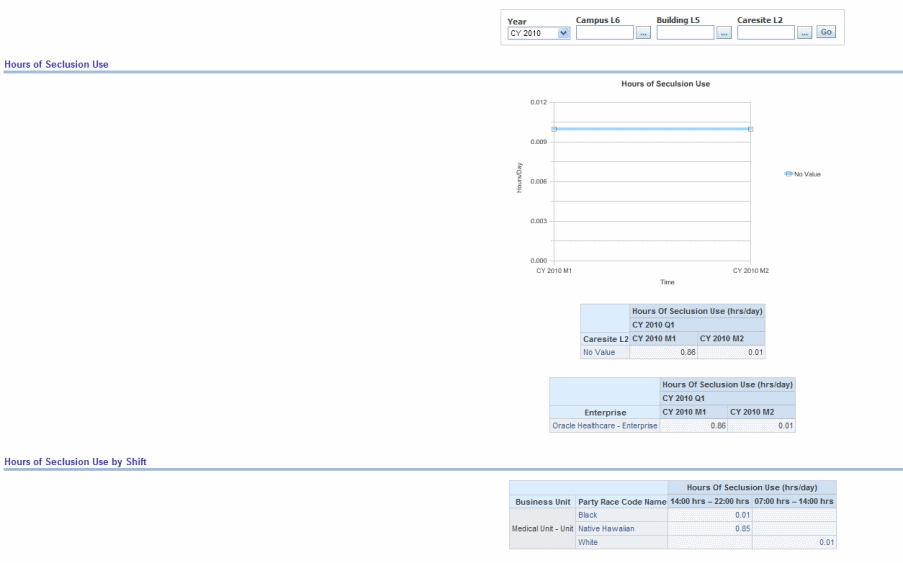
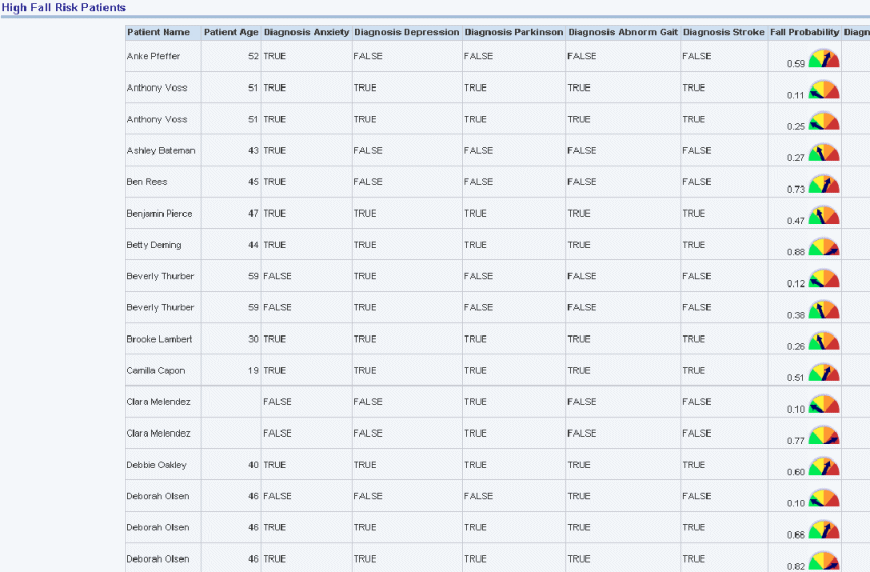
Provides risk assessment sample reports.
Report dimensions are:
Patient Name (DWR_PT)
Patient Age(DWR_PT)
Figure 13-13 Risk Assessment (Clinical): High Fall Risk Patients
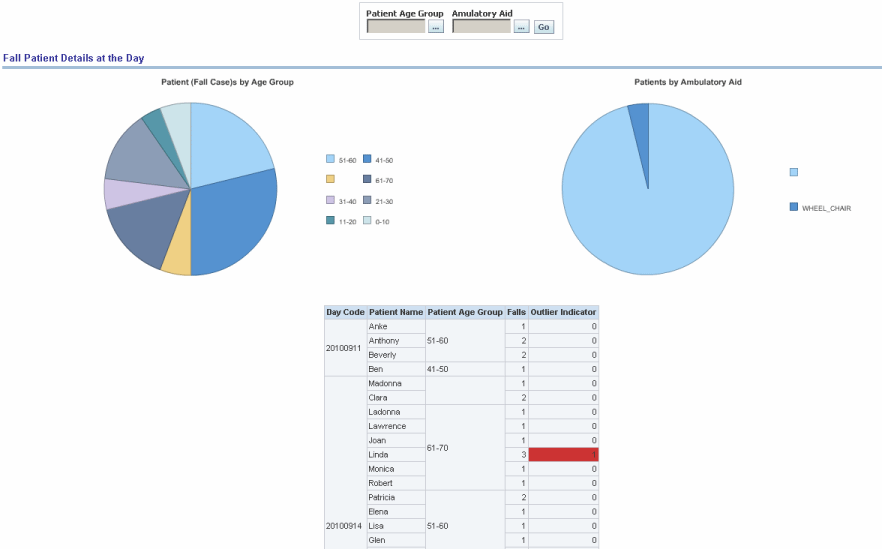
Report dimensions are:
Day Code (DWD_ADVR_EVT_FALL)
Patient Name (DWR_PT)
Figure 13-14 Risk Assessment (Admin): Fall Outlier

Report dimensions are:
Day Code (DWD_ADVR_EVT_FALL)
Patient Name (DWR_PT)
Figure 13-15 Risk Assessment (Admin): Fall Patients Details at the Day
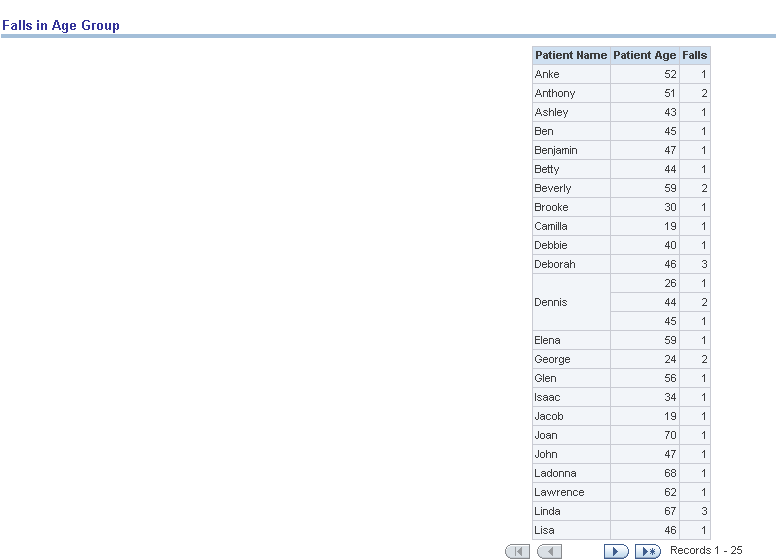
Report dimensions are:
Patient Name (DWR_PT)
Patient Age (DWR_PT)
Figure 13-16 Risk Assessment (Admin): Falls in Age Group
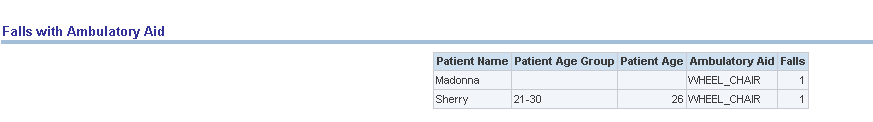
Report dimensions are:
Patient Name (DWR_PT)
Patient Age (DWR_PT)
Figure 13-17 Risk Assessment (Admin): Falls with Ambulatory Aid
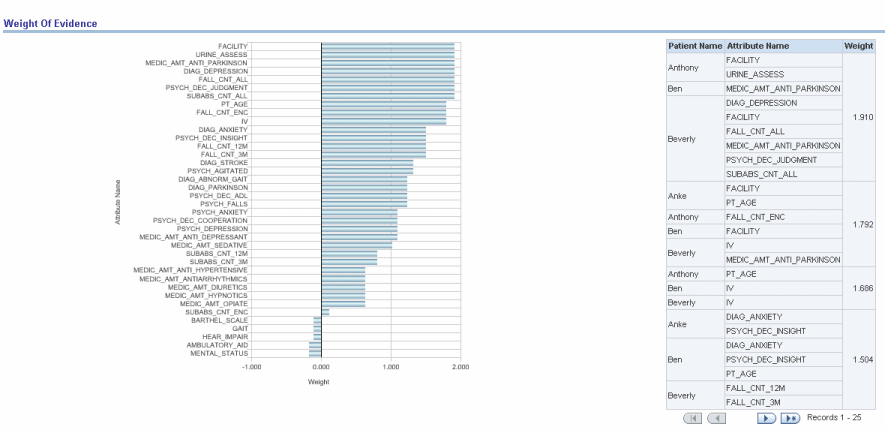
Report dimensions are:
Patient Name (DWR_PT)
Figure 13-18 Risk Assessment (Admin): Weight of Evidence
Provides stroke sample reports.
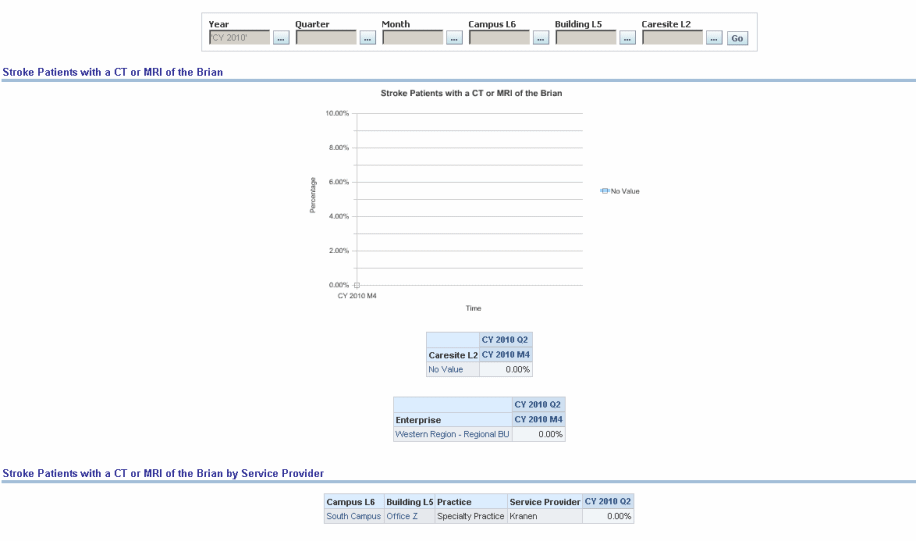
Percentage of final reports for CT or MRI studies of the brain performed either:
In the hospital within 24 hours of arrival, OR
In an outpatient imaging center to confirm initial diagnosis of stroke, transient ischemic attack (TIA) or intracranial hemorrhage
For patients aged 18 years and older with either a diagnosis of ischemic stroke or TIA or intracranial hemorrhage OR at least one documented symptom consistent with ischemic stroke or TIA or intracranial hemorrhage that includes documentation of the presence or absence of each of the following: hemorrhage and mass lesion and acute infarction.
Report dimensions are:
Year (Time)
Quarter (Time)
Month (Time)
Campus (Facility)
Building (Facility)
Caresite (Facility)
Enterprise (Organization)
Figure 13-19 PQRI- Stroke: Stroke Patients with a CT or MRI of the Brain
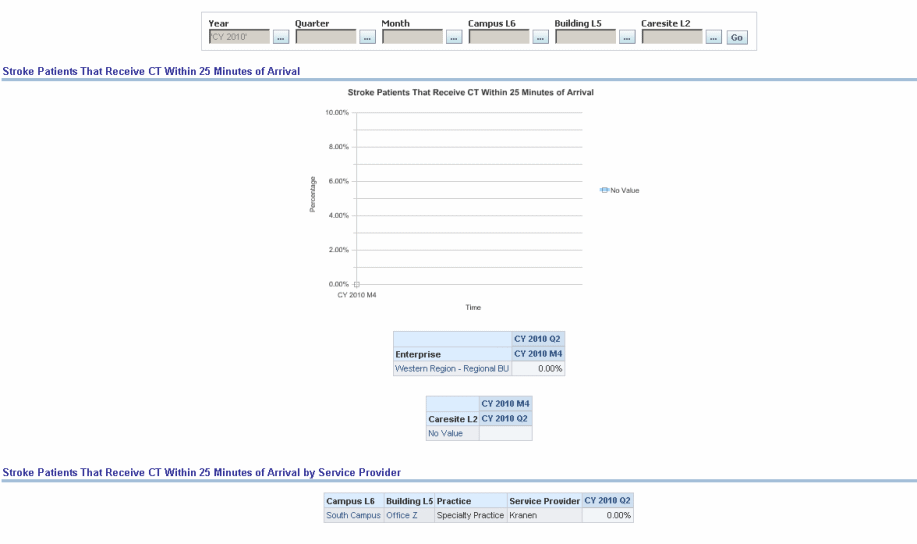
For acute stroke patients. Door-to-CT=25 min: Percent of patients who receive brain imaging within 25 minutes of arrival.
Report dimensions are:
Year (Time)
Quarter (Time)
Month (Time)
Campus (Facility)
Building (Facility)
Caresite (Facility)
Enterprise (Organization)
Figure 13-20 AHA- Stroke: Stroke Patients That Receive CT Within 25 Minutes of Arrival
Provides surgical sample reports.
Percentage of patients aged 18 years and older undergoing isolated Coronary Artery Bypass Graft (CABG) surgery who received a beta-blocker within 24 hours prior to surgical incision.
Report dimensions are:
Year (Time)
Quarter (Time)
Month (Time)
Campus (Facility)
Building (Facility)
Caresite (Facility)
Enterprise (Organization)
Report for this area is similar to that shown in Figure 13-21.
Surgery patients with appropriate surgical site hair removal. No hair removal, hair removal with clippers or depilatory is considered appropriate. Shaving is considered inappropriate.
Report dimensions are:
Year (Time)
Quarter (Time)
Month (Time)
Campus (Facility)
Building (Facility)
Caresite (Facility)
Enterprise (Organization)
Figure 13-21 Surgical: CMS/JCAHO-SCIP Surgery Patients with Appropriate Hair Removal
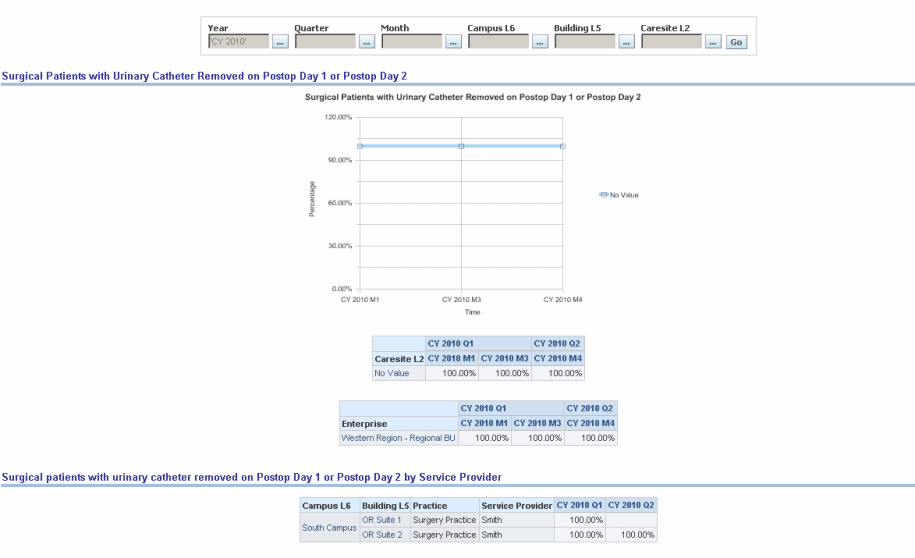
Surgical patients with urinary catheter removed on Postoperative Day 1 or Postoperative Day 2 with day of surgery being day zero.
Report dimensions are:
Year (Time)
Quarter (Time)
Month (Time)
Campus (Facility)
Building (Facility)
Caresite (Facility)
Enterprise (Organization)
Figure 13-22 Surgical: CMS/JCAHO-SCIP Patients with Urinary Catheter Removed on Postop Day 1/2