| Oracle® Health Sciences Cohort Explorer User's Guide Release 1.0 E24437-01 |
|
|
PDF · Mobi · ePub |
| Oracle® Health Sciences Cohort Explorer User's Guide Release 1.0 E24437-01 |
|
|
PDF · Mobi · ePub |
This chapter contains the following topics:
Oracle Health Sciences Cohort Explorer (OHSCE) is an analytical web-based application based on a predefined set of key performance indicators (KPIs), facts, and dimensions with support for predefined and custom reporting. It provides means to analyze all data (primarily clinical and genomics) needed to support the complete biomarker lifecycle starting from data acquisition or discovery through to clinical use at the point of care. OHSCE also functions as a decision support system to monitor process bottlenecks and compliance deviations.
Translational research organizations require insights into key operational and business processes areas that impact translational research performance to evaluate performance of your translational research, and take corrective action to reduce cycle times.
OHSCE integrates translational research data from sources through Oracle Healthcare Data Warehouse Foundation, where appropriate, into a predefined data warehouse schema and generates both predefined and custom reports of key metrics across the translational research spectrum. OHSCE lets you perform the following functions:
Extract all necessary data from sources through Oracle Healthcare Data Warehouse Foundation into a predefined data warehouse, for viewing through the rich dashboard and report interface of Oracle Business Intelligence Enterprise Edition Plus (OBIEE).
View predefined analytical reports delivered with the application.
Utilize simple to use interface with over 350 filtering criteria to rapidly assess patient cohort counts across many patient dimensions without having to write a single line of SQL.
Rapidly create new reports using the extensive predefined cohort metrics across patients' clinical and genomics data.
Figure 1-1 The Oracle Health Sciences Cohort Explorer Architecture
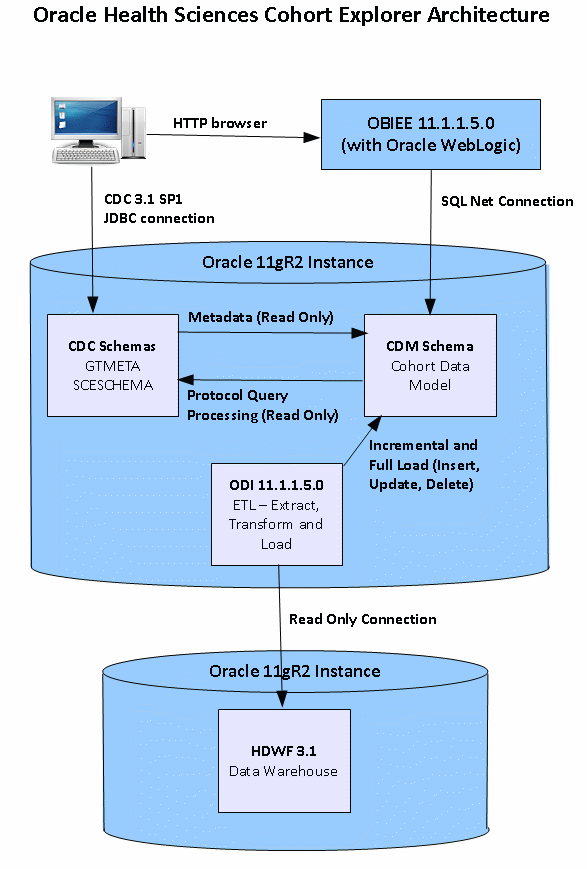
The OHSCE architecture includes the following principal components:
A predefined Intelligence application, based on Oracle Business Intelligence Enterprise Edition Plus (OBIEE), including Oracle BI Presentation Services, and a predefined set of Oracle BI reports, accessible through prebuilt interactive dashboards.
A protocol editor in Oracle Health Sciences Clinical Development Center.
A prebuilt clinical data warehouse schema, designed to drive both predefined and custom intelligence reports.
Extract Transform Load (ETL) programs, designed to extract from HDWF databases into Cohort datamart schema.
A predefined presentation catalog including a unique mix of cohort measures (facts, dimensions), populated by the data warehouse schema.
Oracle Health Sciences Cohort Explorer data model comprises of a set of tables while the ODI repository comprises of a set of ODI interfaces. When you install the data model and ODI repository and also run the ODI interfaces the cohort data model will be loaded with patient related medical information from the Healthcare Data Warehouse Foundation (HDWF).
Once the data model is loaded with patient information, it can be used for reporting and analytical purposes.
Protocols are predefined inclusion and excision criteria. For cohort, there are two levels of filters; the first level you filter data using a predefined protocol which can be edited in Oracle Health Sciences Clinical Development Center (CDC). At the report level you can filter data by selecting prompts on the dashboard page.
When you run the master install script, it creates two roles Protocol_Admin (all privileges for protocol query folder) and Protocol_User (has only read -only privileges.)
The script also creates Protocol Queries folder (workbook). You need to create all protocol queries under this workbook.
Login into CDC as admin user
When you create a new query, drag and drop relevant tables from CDM cartridge to create protocol query. For information about query operations, refer to Oracle Health Sciences CDC User Guide PF_CDC_CDR_User_Guide.
Once the ETL has evaluated inclusion of patients for each protocol query, you can use the protocol dimensions as filters in OBIEE.
Note:
Protocol_Admin role should be used to create, modify or delete queries and Protocol_User can only view results of query in read-only mode.OHSCE provides reports for the following key functional areas:
Rapidly assess how many patients qualify for a given study.
View details of the patient cohort down to the individual patient's medical history without revealing patient's identity.
Evaluate the operational efficiency of your organization via the operational dashboards in the Summary Dashboard page.
Asses how many biospecimen samples are currently available for a given set of patients.
Use intuitive prompts to control patient cohort lists enabling you to add or remove patients from a cohort.
Get a glimpse of patient's medical history events and their relative timelines for cohort patients within a temporal bar display report.
See Also:
Oracle Health Sciences Cohort Explorer is developed in compliance with HIPAA regulations to protect any patient identifiable medical information and follows the software development guidelines and requirements for FDA 21 CFR Part 11 compliant software.
The origin of any data stored in CDM must be traceable to its source, and all transformations applied to the data must be accessible. Data sourced from HDWF is traced by the following criteria:
ETL Load: When the data was loaded from HDWF into CDM.
ODI Interfaces: The version of ODI interface used to transform the data from the HDWF to CDM, and when it was executed.
Configuration Seed Data: There are two tables which contain seed data. Based on these tables, data is loaded in CDM and another configuration table, which is automatically seeded during ETL load.
Data in Oracle HDWF also keeps audit trails of all modifications.
You can use a third-party versioning tool or the in-built functionality of ODI versioning to manage ETL versions. Currently all ODI objects are in the default version.