| Agile Product Lifecycle Management Agile Recipe Management for Pharmaceuticals Recipe Management Guide Release 9.3.4 E39303-02 |
|
 Previous |
 Next |
| Agile Product Lifecycle Management Agile Recipe Management for Pharmaceuticals Recipe Management Guide Release 9.3.4 E39303-02 |
|
 Previous |
 Next |
The Recipe Management module within the Agile PLM Recipe & Material Workspace (RMW) solution provides integrated recipe authoring capabilities that help you to track the development process of a product. In RMW, recipes are integrated with the related Bill of Materials (BOM), Bill of Equipment (BOE), and Bill of Processes (BOP) to maintain a complete and reliable electronic record.
The primary benefits of using RMW for recipe management are:
Re-usability of recipe objects - Once created, each recipe object can be reused in other recipes and experiments or work requests, across multiple sites.
Recipe and experiment results captured in electronic format - Recipes, experiments, and bills need not be created manually (in paper format) for each recipe instance.
Ability to import and export recipes in XML - Eliminates manual errors during tech transfers; the transfer can be completed in a fraction of the usual time.
Greatly reduced effort in authoring - Easy-to-use graphical and text editors simplify and expedite the recipe authoring process.
This guide provides information on all the features and functionality of the RMW Material Management module. It also covers instructions on how to use the various menus and commands available on the RMW User Interface to create and manage material objects. The features that are visible to you on the interface are determined by the access privileges assigned to you by an administrator.
The complete list of RMW manuals is provided here for the benefit of users and administrators of the RMW solution.
Getting Started with Agile Recipe Management for Pharmaceuticals - describes common concepts, basic navigation, searches and workflows. Also covers how to work with reports, standards, and environmental conditions.
Agile Recipe Management for Pharmaceuticals Administrator Guide - describes all administration and configuration information including Agile PLM integration requirements.
Agile Recipe Management for Pharmaceuticals - Process Management Guide - describes the features of the Process module, covering the creation and execution of projects and campaigns, control recipes, and work requests.
Agile Recipe Management for Pharmaceuticals - Recipe Management Guide - describes the features of the Recipe module, covering the authoring and management of recipes and recipe templates.
Agile Recipe Management for Pharmaceuticals - Material Management Guide - describes the features of the Materials module, covering how to work with material requests, inventory, and allocation. Also covers how to manage analytical activities.
Agile Recipe Management for Pharmaceuticals -Equipment Management Guide - describes the features of the Equipment module, covering equipment qualification, loan, lease, and reservation.
Agile Recipe Management for Pharmaceuticals - Import/Export Guide - describes how to export and import RMW business and administrator objects from a source system to a target system.
RMW is accessed only through the Agile PLM user interface. Refer to Getting Started with Agile PLM along with the Agile PLM Administrator Guide for a thorough understanding of PLM processes. The complete set of Agile PLM documentation, including RMW documentation, is available on the Oracle Technology Network (OTN) Web site http://www.oracle.com/technetwork/documentation/agile-085940.html.
A Recipe contains the minimum set of information about the developmental requirements of a specific product. Recipes generally include instructions about gathering and combining raw material, the type of equipment to be used, and processes to be followed to create the target product.
In RMW, instructions in a recipe can have embedded variables (placeholders) for generic resource names - for resources such as equipment, material, or standards. This type of authoring ensures that the recipes can be reused as required, with the variables resolved to specific values for each use in a project or campaign.
Within the product development cycle for a target material, as a project or campaign gets underway, the recipe that defines the process of creating that product is attached to a process step. Generic recipes are converted to project-specific Control Recipes, and these are in turn used to create Work Requests. Work Requests can be completed in an experiment, or logged as a production batch record.
Recipes can be versioned based on feedback from the pilot plant or commercial operations.
Recipes are classified into the following types:
General Recipe: A General Recipe is the basis for lower-level recipes and used at the company level. It specifies the raw materials, quantities (of raw material) required and processing information for making the product. It only communicates processing requirements to multiple manufacturing sites.
Site Recipe: A Site Recipe is created directly and takes into consideration the specific conditions or constraints of a particular manufacturing site. The user can select multiple sites for a site recipe. It provides the level of detail necessary for site-level, long-term production scheduling.
Site recipes contain information tailored for a target location. These can be modified for local language, local measurements and availability of local raw materials and include information about on-site processing, storage capacity, and constraints. From a general recipe, you can derive multiple site recipes, each covering a part of the general recipe that may be implemented at a specific site.
Master Recipe: A master recipe is targeted to a processing area and is created directly. In this recipe creation, user can select only one recipe. Master recipes depend on equipment types or classes, such as a glass-lined reactor or mixing vessel. These recipes can contain product-specific information required for detailed scheduling, such as equipment requirements. But unlike the general and site recipes, S88 batch control requires a master recipe. A master recipe is the template for recipes used to create individual batches. Without this template, no specific batch recipes can be created, and therefore, no batches can be produced.
Control Recipe: A control recipe is used to create a single, specific batch. It starts as a copy of a master/general/site recipe and is modified as necessary to create a batch. The modifications may account for batch size, characteristics of raw materials on-site (For example: potency), or actual equipment to be used. While several (dozens, hundreds, or thousands of) batches may use the same master recipe, every batch has a single control recipe unique to that batch and that batch alone.
Two control recipes may be identical in ingredients, quantities, or equipment used, but they are identified individually. Control recipes unique to individual batches allow product tracking or genealogy tracking to occur.
For complete information on Control Recipes, see Recipe & Material Workspace Process Management Guide.
The difference between a Recipe and a Recipe Template is that templates have unresolved variables and parameters, with undefined constraints. A Recipe needs to be complete and ready before you can execute it.
A Recipe in RMW is a structured compilation of a set of components as shown below. The following sections describe each component.
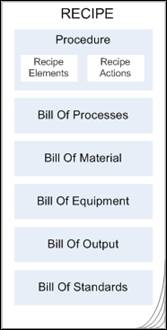
In RMW, a recipe procedure is a structural representation of Recipe Elements, Recipe Actions, and associated objects. The RMW user interface allows you to create Recipe Element Templates and Recipe Action Templates which can then be reused while authoring recipes.
A recipe element is a procedural element that is used to represent an entity in procedure function charts. A recipe element can be any of the following:
Unit Procedure - an ordered set of operations carried out to completion on a single unit. It is a contiguous production sequence acting on a single unit only. Only one unit procedure is allowed to be active on a unit at a time. Multiple unit procedures can run concurrently as part of the same procedure, as long as they are active on different units. Unit procedures can contain operations, phases and actions.
Operation - an ordered set of phases carried out to completion within a single unit. An operation usually takes the material being processed through some type of physical, chemical, or biological change. Like unit procedures, the standard presumes only one operation is active on a particular unit at a time. Operations can contain phases and actions.
Phase - A phase is the smallest element of procedural control that can accomplish process-oriented tasks. A phase performs unique and generally independent, basic process-oriented functions, such as charging an ingredient or agitating a tank. All other elements (procedures, unit procedures, and operations) group, organize, and direct the phases.
A Recipe Action contains an instruction to perform a unique and generally independent action related to the manufacturing process. It allows the author to specify the following details:
Variables - resources you can use. For example: Equipment, Material, and Standards
Parameters - parameters you need to measure or control
Second Person Verification - whether you require electronic signature and second person verification.
Electronic Signature indicates that an operator completed the task
Second Person Verification indicates that a second person or automation verified the action or measurement
Execution mode - manual or automatic.
A BOP for a target material contains a list of materials, equipment, process steps, standards, control parameters, and measured parameters which may use assays. BOP contains the process of sequential flow of elements and actions. At the heart of the creating a Work Request is a series of steps that go into creating the target material, called the Bill of Process (BOP).
BOP is composed of one or more recipe elements and/or recipe actions. Recipe elements contain recipe actions which is the lowest level of instruction.
A Bill of Material (BOM) is an assembled list of all materials and their quantities (with UOM's) required to produce a target/output material at a given site, including materials not related to production. For example, shipping materials, or other consumables required for the overall recipe.
Recipes contain text instructions embedded with variables which act as placeholders for defined material types or categories. Each variable is associated with certain criteria that qualify the material. When these recipes in the master library are "resolved" by the user, the software automatically generates the BOM for the Recipe.
While editing a Recipe Action instance in edit Recipe page user can add multiple Material directly.
A Bill of Equipment (BOE) is the approved list of equipment used in the process of creating a product. When the equipment-related variables in recipes are resolved by the user, the application generates the BOE for the Recipe.
While editing a action instance in edit recipe page, user can add Equipment directly.
A Bill Of Assays (BOA) is a list of assays that are referenced indirectly from a Recipe. If recipe actions within a recipe contain parameters that have references to defined assays in the system, the application generates a BOA for the Recipe.
A Bill of Standards (BOS) is a library of pre-defined statements relating to the safety and caution of a manufacturing process. It comprises of general instructions, guidelines and specifications you have to adhere to while handling equipment, material and processes in the industry and organization. Standards are used while authoring Unit Operations and creating ControlRecipes, Process Steps and Work Requests.
Quantities of materials or resources, created as a result of the product manufacturing processes define Output. The quantity specified for output material at Recipe creation level is the planned quantity. Actual quantity will be added only after the output material is added to inventory at Work Request level.