| Oracle® Argus Safety BIP Aggregate Reporting User's Guide Release 8.1 E76742-01 |
|
|
PDF · Mobi · ePub |
| Oracle® Argus Safety BIP Aggregate Reporting User's Guide Release 8.1 E76742-01 |
|
|
PDF · Mobi · ePub |
This chapter contains the following topics:
The Post-Marketing Aggregate Report (PMAR) includes OOB line listings and summary tabulations that can be used to meet supplemental line listings for PBRERs.
The Post Marketed Aggregate Report includes the following line listing sections:
Main line listing
Ad hoc 1 line listing
Ad hoc 2 line listing
Ad hoc 3 line listing
Ad hoc 4 line listing
The PMAR includes the following Summary tabulations:
Summary Tabulation for HCP
Summary Tabulation for Consumer
Summary Tabulation for Clinical Trials
Cumulative Summary Tabulation for Serious Unlisted Events
The following Information is part of the PMAR Trailer Section:
Case IDs without any qualifying drugs
Case IDs without any qualifying events
Labels configured for drugs in the drug list
Cases with Missing Assessment
The PMAR considers parameters as per the sheet available in the PBRER specifications section for the column Required in PMAR? marked as Yes.
PSUR Configuration: as per the attached sheet Related Argus Configuration.
BIP report parameters: as per attached sheet Parameter Display.
To support PMAR, a main case series and cumulative case series is generated based on the inclusion criteria defined in the PSUR configuration of Argus Safety. The system evaluates the list of cases from the main and cumulative case series to categorize them.
Name
The PSUR configuration screen lets you distinguish the configuration options used in the BIP report as parameters only if the BIP Aggregate Reporting module is enabled.
Refer to the section Section 2.4, "Periodic Configuration Identification of BIP Parameters," for the report parameters used in PMAR.
Cases printed in the Main line listing are based on the Main case series if specified; otherwise they use the Inclusion Criteria and Product Selection parameters of the PSUR configuration.
The Main Line Listing provides grouping based on the following groups of cases:
Table 8-1 Main Line Listing Grouping
| Sl | Temp table reference |
|---|---|
|
Grouping based on HCP or Consumer |
Cases having Primary reporter marked as Health care Professional are considered as HCP cases. Cases having Primary reporter not marked as Health care Professional are considered as Consumer cases. |
|
Grouping based on Initial or Follow-up |
Cases are listed under Initial or Follow-up based on temp table logic. Initial case listing is printed followed by Follow-up cases. |
|
Grouping based on Products |
Cases with company suspect products matching those in the Products selected are grouped. If there are no cases for a product specified in the product selection specified in Report Configuration, this product name does not appear as a header. If a case has multiple company suspect products that are in the product selection specified in report configuration, the case is presented in each product's section; that is, the case appears multiple times in the report. |
|
Grouping based on SOC (as per event order specified in the case form event tab) |
Cases are grouped by the SOC of the Event. The system displays the SOC group header in the internationally agreed order as specified in the codelist SOC_DISPLAY_ORDER. Case appears under the SOC of the Primary event and the system prints a reference of this information for SOCs of other events if the BIP Report parameter List cases in the line listing under SOC for each diagnosis is Y and List Cases under all events, details under the primary event is selected. Case appears under the SOC of the Primary event and not under other SOCs if the BIP Report parameter List cases in the line listing under SOC for each diagnosis is N and List Cases only once, under the primary event is selected. |
|
Grouping based on Case Seriousness |
Cases with Serious case level seriousness are printed under the group header Serious. Cases with Non-Serious case level seriousness are printed under the group header Non-Serious. Cases with case level seriousness as blank are printed under Seriousness not defined. The order of printing these group headers is Serious, Non-Serious and Seriousness not defined. |
|
Sorting based on Case Numbers |
Cases are displayed in the ascending order of the Case Numbers in the Main line listing. |
Table 8-2 Main Line Listing Columns
| Column Name | Field Description |
|---|---|
|
Case Number |
If the BIP report parameter Indicate if case was expedited previously is Y, the symbol specified in the BIP parameter Symbol for Expedited cases appears as a superscript. A footnote appears at the end of the page as: <symbol>: Expedited Case. If the BIP report parameter Indicate if case was expedited previously is Y, the footnote for Expedited Cases appears even though there may not be any cases marked as Expedited. |
|
Country |
Country of Occurrence. |
|
Report Type |
Report type is displayed from the flex bucketing codelist field RPTTYEPGRP for the report type entered in the case. |
|
Unique PatientID |
Unique Patient ID as per the BIP report configuration parameter UNIQUE_PATIENT_ID_FORMAT, which has the following options: Pt - Patient ID CePt - Center ID, Patient ID InPt - Investigator Name, Patient ID StCeInPt - Study ID, Center ID, Investigator Name, Patient ID StCePt - Study ID, Center ID, Patient ID StCnCeInPt - Study ID, Country name, Center ID, Investigator Name, Patient ID StCnCePt - Study ID, Country name, Center ID, Patient ID StCoCeInPt - Study ID, Country ISO Code, Center ID, Investigator Name, Patient ID StCoCePt - Study ID, Country ISO code, Center ID, Patient ID StInPt - Study ID, Investigator Name, Patient ID |
|
Sex |
Patient Sex. PATIENTSEXTEXT is populated using the decode context GL of the PATIENTSEXCODE field. |
|
Age |
Patient Age; if this data is missing, the Patient age group is printed. |
|
Company Suspect Products |
Company suspect products that match the Product specified in the Report configuration. If the BIP report parameter Print Unblinded Data is Y, the system prints the Unblinded Study Product. If the BIP report parameter Print Unblinded Data is N, the system prints the Blinded Study name instead of the actual study product. |
|
Strength |
Strength and Strength units. |
|
Dosing Regime |
If the Report parameter Print Dose Text in place of Regimen Dose is checked, then Regimen Dose data is printed. Otherwise, Daily dose and Route data are printed with the respective company suspect drug based on the BIP report parameter Dosage String Format with the options: Do - Print Dose Only. DoFo - Print Dose, and Formulation. DoFoFr - Print Dose, Formulation, and Frequency. DoFoFrRt - Print Dose, Formulation, Frequency, and Route of administration. DoFoRt - Print Dose, Formulation, and Route of administration. DoFr - Print Dose, and Frequency. DoRt - Print Dose, and Route of administration. Multiple components are separated by semicolons. If a suspect drug has multiple dosage regimens, the dosage information for each record is printed in a separate line. |
|
Action Taken |
Action taken is printed for the company suspect drug. |
|
Treatment Date |
Dates of the First and Last dose are printed in DD-MON-YYYY format. |
|
Treatment Duration |
Dosage duration is printed with units. If duration is not available, the text entered for the BIP parameter Default for NULL Values is printed. |
|
Event Start and Stop Date |
Event Onset and Stop dates are printed for the corresponding Reactions in the DD-MON-YYYY format. |
|
First Dose to Onset |
Onset Latency data is printed for the corresponding suspect product and events. |
|
Outcome |
Event Outcome is printed for the corresponding events. If Event Outcome is not entered, nothing is printed. |
|
Event as Reported (Preferred term) |
Description as Reported and LLT or PT of Event is printed in the format: <Desc. As reported> (<LLT or PT>). If Description as Reported is not entered, LLT or PT is printed with brackets. LLT or PT are based on the BIP report parameter Print LLT instead of PT. If the BIP Report Parameter Print only the Term (LLT or PT) is Y, only the LLT or PT is displayed. Description as reported is not printed. The system prints the Diagnosis if the BIP report parameter Use Only Diagnosis Events is Y. If this parameter is N, the Diagnosis and Symptoms are printed. The system prints diagnosis and symptoms separately and marks them clearly using symbols provided in the BIP parameter Symbol for Diagnosis Symptoms, if the BIP report parameter Print Diagnosis Symptoms Separately is Y. If this parameter is N, then they are printed without any identification symbol. For Unlisted events, the system prints the text specified in the BIP parameter Symbol for Unlisted Events. For SUSAR events, the system prints the text specified in the BIP parameter Symbol for SUSAR Events. For events marked as Special Interest Events in the Report parameter, the system prints the text specified in the BIP parameter Symbol for Special Interest Events. |
|
Causality Rpt/Cmp |
Causality as per reporter and Company are printed for the corresponding Product and Events in the format: <Causality as per reporter> / <Causality as per company>. |
|
Seriousness |
Event seriousness is printed against each event. Serious, Non-Serious, and Seriousness not determined are printed based on the data in CASE_EVENT.SERIOUSNESS. |
|
Listedness |
Listedness information of the event. Listedness is printed based on the BIP Report parameter Labeling Algorithm if the PMAR is executed from BIP. If the PMAR is executed from Argus Safety, the following report parameters are considered: Use Assessment in Cases. Re-assess cases against datasheet in effect at beginning. Re-assess cases against datasheet in effect at end. |
|
Line listing Comments |
Text from the Company comment fields. |
|
Other Medications |
The list of all suspect products and concomitant products in the case, except the drug for which the report is being run, is printed in the following format: Other suspect Products: <Other suspect Products> Concomitant Medication: <Concomitant Products> |
|
Death date and cause of death (Verified ? Y/N): |
Patient Death date and Cause of death are printed in the following format only for Death cases: Death date and cause of death (Verified? Y/N): DD-MON-YYYY <Cause of death > (<Y/N>) For example, Death date and cause of death (Verified? Y/N): 01-JAN-2014 cholera(Y). If cause of death is populated from autopsy results, then cause of death verified is Y; otherwise it is N. |
The following counts are provided in the Main line listings at the end of various groupings:
Count of Cases for Initial or Follow-up based on HCP or Consumer and Drug appear in the following order:
Count of Cases for Initial / Follow-up based on HCP /Consumer and <Drug>
Count of Cases for Initial based on HCP and <Drug>
Count of Cases for Follow-up based on HCP and <Drug>
Count of Cases for Initial based on Consumer and <Drug>
Count of Cases for Follow-up based on Consumer and <Drug>
Count of Cases for Initial or Follow-up HCP appear in the following order:
Count of Cases for Initial HCP
Count of Cases for Follow-up HCP
Count of HCP or Consumer Cases appear in the following order:
Count of HCP Cases
Count of Consumer Cases
The following is the PMAR Report format:
Ad hoc section has the same line listing format and fields as the Main line listing section.
Ad hoc 1 line listing is based on cases from ad hoc Case List 1.
Ad hoc2 line listing is based on cases from ad hoc Case List 2.
Ad hoc3 line listing is based on cases from ad hoc Case List 3.
Ad hoc4 line listing is based on cases from ad hoc Case List 4.
Counts that are displayed in the ad hoc section are the same as those in Main line listing.
Cases considered for Summary Tabulations section are based on the Main case series.
The PMAR supports the following tabulation sections:
PMAR Summary Tabulation
Summary Tabulation for Consumer Cases
Summary Tabulation for Clinical Cases
Cumulative Summary Tabulation for serious unlisted events
HCP and Non-HCP cases are tabulated in this section of the report. The table is organized first by Initial or Follow-up, Product and SOC, then by Solicited or Non Solicited, Seriousness, Study Type (Sponsored versus Unsponsored Study).
Only related events (related to company drug that is specified in product selection) are considered for this tabulation for solicited cases. Relatedness information is not considered for Non-Solicited cases.
If the report parameter Exclude non serious cases from summary tabulations is set to Y, the system still prints the Grouping and Counts based on Non-Serious events which are part of serious cases. Only Non-serious cases and corresponding events are ignored based on parameter value of Y.
Summary Tabulations for HCP cases report are grouped on the following options:
| SI | Temp Table Reference |
|---|---|
|
Grouping based on Initial or Follow-up |
Cases are listed under Initial or Follow-up based on temp table logic. If the report parameter Include Follow-up cases from Summary Tabulation is set to N, then summary tabulations are not printed for follow-up cases. |
|
Grouping based on Products within Product Selection |
Cases with the company suspect products matching with the products selected in the report configuration are grouped. Drug names that appear in the group header are arranged in ascending order. If there are no cases for a drug that is specified in the drug list, then this drug name does not appear as a header. If a case includes multiple company suspect drugs that are in the drug list, the case is presented in each drug's section; the case appears multiple times in the report. |
|
Grouping based on SOC |
Cases are grouped by the SOC of the Primary Event. SOC group headers are displayed in the Internationally agreed order as specified in the codelist SOC_DISPLAY_ORDER. If the Report parameter List cases in the Line Listing under SOC for each diagnosis is Y, the case is listed multiple times under different SOCs. If this parameter is set to N, the case is printed under the SOC of the primary event, and a reference to this information is printed for the SOCs of other events. |
Table 8-4 HCP Summary Tabulation Columns
| SI | Temp table reference |
|---|---|
|
Preferred Term |
LLT or PT is printed based on the report parameter Print LLT instead of PT. System prints diagnosis if the report parameter Use Only Diagnosis Events is Y. If this parameter is N, then diagnosis as well as symptoms are printed. LLT or PT is arranged in alphabetical order within a SOC. |
|
Solicited |
Solicited cases are clinical trial cases having atleast one event related to the drug. Either the company or reporter causality flag is Y. |
|
Serious-Sponsored |
The count of Serious Events with report type group as Sponsored Study, and case type text as Solicited is printed in this column. |
|
Serious-Non-Sponsored |
The count of Serious Events with report type group as Non-Sponsored Study, and case type text as Solicited is printed in this column. |
|
Sub-total |
The sub-total is computed by summing-up Serious sponsored and unsponsored events from Solicited cases. |
|
Non-Solicited |
Non-Solicited cases are Non-Clinical trial cases having atleast one event related to the drug. Either the company or reporter causality flag is Y. |
|
Serious-Direct to MAH |
The count of Serious Events with report type group as Direct to MAH, and case type text as Non-solicited is printed in this column. |
|
Serious-HA |
The count of Serious Events with report type group as HA, and case type text as Non-solicited is printed in this column. |
|
Serious-Stimulated |
The count of Serious Events with report type group as Stimulated, and case type text as Non-solicited is printed in this column. |
|
Serious-Literature |
The count of Serious Events with report type group as Literature, and case type text as Non-solicited is printed in this column. |
|
Sub-total |
The sub-total is computed by summing-up Serious events from Non-Solicited cases. |
|
Non-Serious -Direct to MAH |
The count of Non-Serious Events with report type group as Direct to MAH, and case type text as Non-solicited is printed in this column. |
|
Non-Serious-HA |
The count of Non-Serious Events with report type group as HA, and case type text as Non-solicited is printed in this column. |
|
Non-Serious-Stimulated |
The count of Non-Serious Events with report type group as Stimulated, and case type text as Non-solicited is printed in this column. |
|
Non-Serious-Literature |
The count of Non-Serious Events with report type group as Literature, and case type text as Non-solicited is printed in this column. |
|
Sub-total |
The sub-total is computed by summing-up Non-Serious events from Non-Solicited cases. |
|
Total |
The total is computed by summing-up the sub-totals of Solicited and Non-solicited columns. |
|
Total events for <SOC> |
The count of Events is printed against respective column at the end of SOC grouping. |
|
Total events for <Drug> |
The count of Events is printed against respective column at the end of Drug grouping. |
|
Total of distinct cases for <Drug> |
The count of Distinct Cases is printed against respective column at the end of Drug grouping. |
Figure 8-2 Format for HCP Summary Tabulations
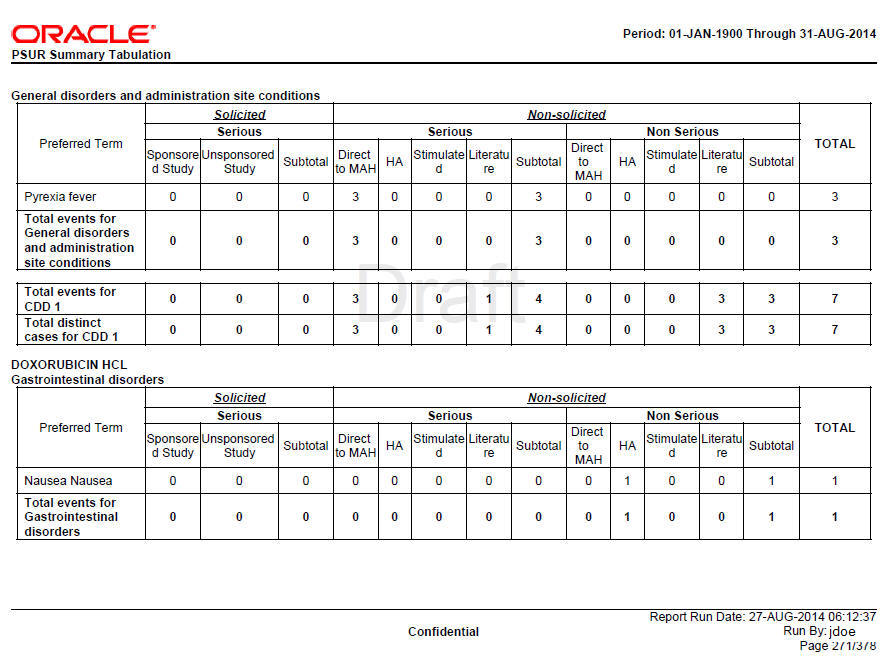
Format for HCP Summary Tabulations
Consumer cases are tabulated in this section of the report. The table is organized first by Initial or Follow-up, Product and SOC, then by Seriousness.
Cases with GTT_RPT_AGG_CASE.CASEMEDICALLYCONFIRMTEXT = Consumer are considered for this section, that is, consumer cases are those cases where the primary reporter has Health Care Professional set to No.
This section is not printed if Include only HCP cases if Summary Tabulation is set to N.
Grouping
Summary Tabulations for Consumer cases reports are grouped on the following options:
Grouping based on Initial or Follow-up
Grouping based on Products within Product Selection
Grouping based on SOC
Grouping logic is as per requirements defined in Summary Tabulation for HCP Cases.
Consumer Summary Tabulation Columns
Table 8-5 Consumer Summary Tabulation Columns
| SI | Temp table reference |
|---|---|
|
Preferred Term |
LLT or PT is printed based on the report parameter Print LLT instead of PT. System prints diagnosis if the report parameter Use Only Diagnosis Events is Y. If this parameter is N, the Diagnosis and Symptoms are printed. LLT or PT is arranged in the alphabetical order within a SOC. |
|
Serious |
The count of Serious Events from Consumer cases grouped by Initial or Follow-up, Product SOC, is printed in this column. |
|
Non-Serious |
The count of Non-Serious Events from Consumer cases grouped by Initial or Follow-up, Product SOC, is printed in this column. |
|
Total (Column) |
The total is computed by summing-up Serious and Non-Serious events against individual LLT or PT. |
|
Total (Row) |
The total is computed by summing-up Serious and Non-Serious events; Grand total of all events is computed by summing-up all serious and non-serious events. |
Figure 8-3 Consumer Summary Tabulation Screen
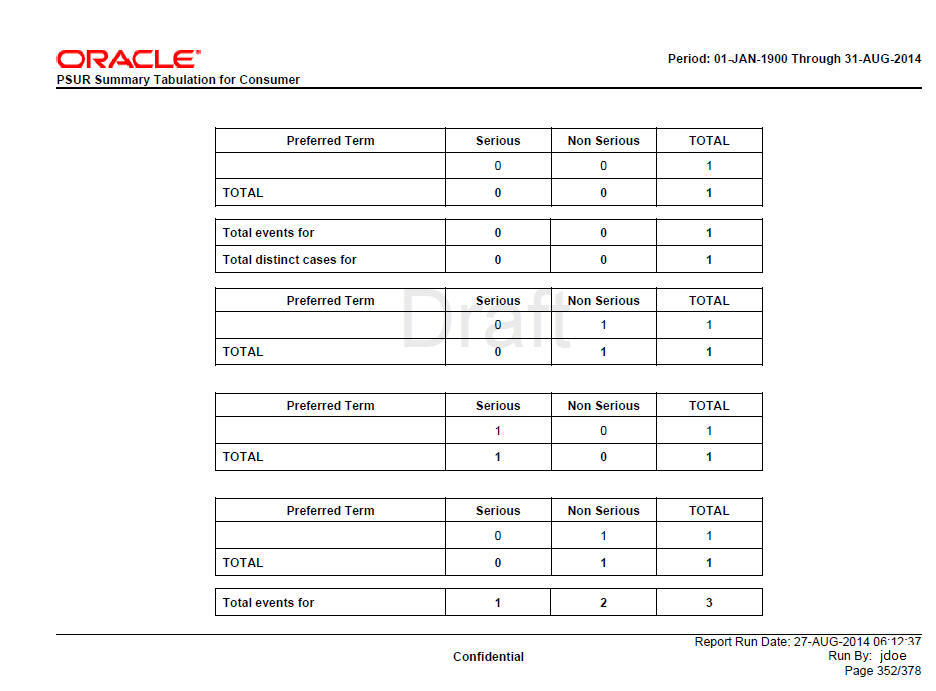
The Cumulative Summary Tabulations section is derived based on the Cumulative case series for Cumulative period event counts and Main case series for Current period event counts.
This tabulation includes only the S or UL Diagnosis events (relatedness of an event is not considered for this tabulation).
This table is organized first by HCP or Consumer, Product, and SOC, then by a comparison of events from current reporting with the cumulative reporting report.
Grouping
Cumulative Tabulations for Serious Unlisted (S or UL) cases report are grouped on the following options:
Grouping based on HCP or Consumer
Grouping based on Products within Product Selection
Grouping based on SOC
Grouping logic is as per requirements defined in Summary Tabulation for HCP Cases.
Clinical Trial Summary Tabulations
Table 8-6 Clinical Trial Summary Tabulations
| SI | Temp table reference |
|---|---|
|
Preferred Term |
LLT or PT from S or UL diagnosis events is printed based on the report parameter Print LLT instead of PT. LLT or PT is arranged in the alphabetical order within an SOC. |
|
Current Period |
The count of S or UL diagnosis events from the Main case series is computed against LLT or PT. Follow-up events from the Main case list are not considered for the Current Period. |
|
Cumulative Period |
The count of S or UL diagnosis events from the Cumulative case series is computed against LLT or PT. |
|
Total |
Summation of count of diagnosis event within SOC is printed under Current Period and Cumulative Period respectively. |
|
Overall total diagnosis for <Product> |
The count of diagnosis is printed against respective column at the end of Product grouping. |
|
Overall total of distinct cases for <Product> |
The count of Distinct Cases is printed against respective column at the end of Product grouping. |
Figure 8-4 Summary Tabulation for Clinical Trials Screen
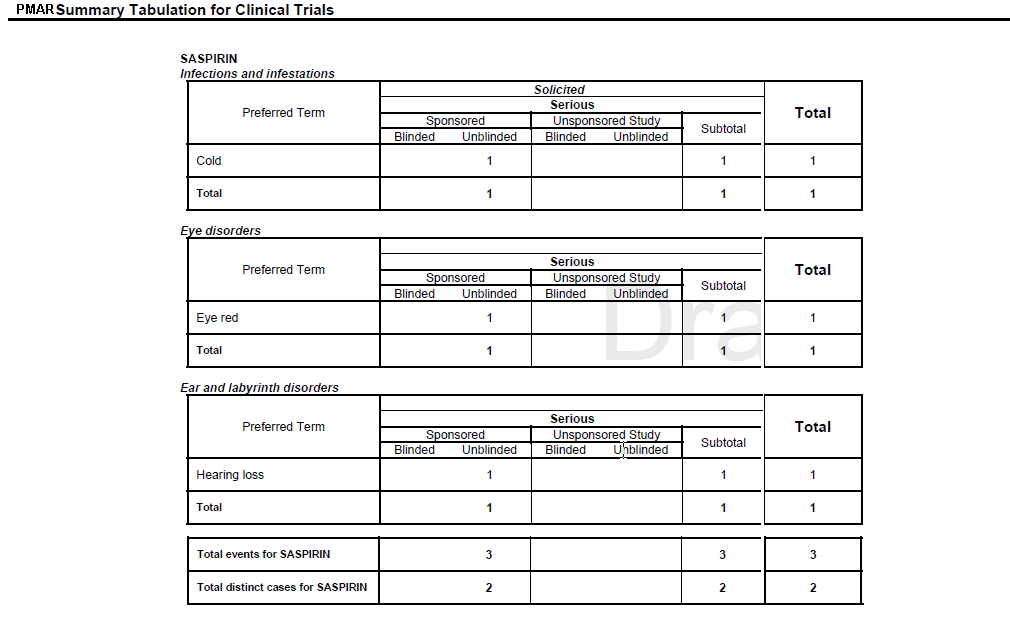
Refer to Section 6.6, "Executing the PBRER from BIP" to execute a PMAR from BIP.