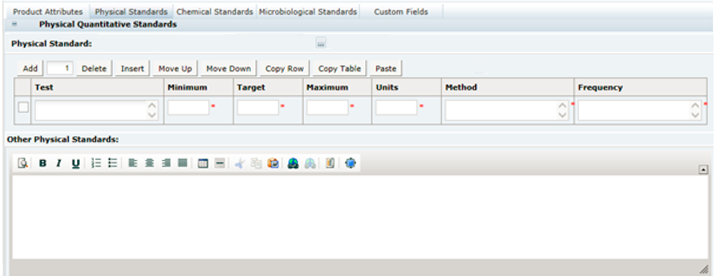
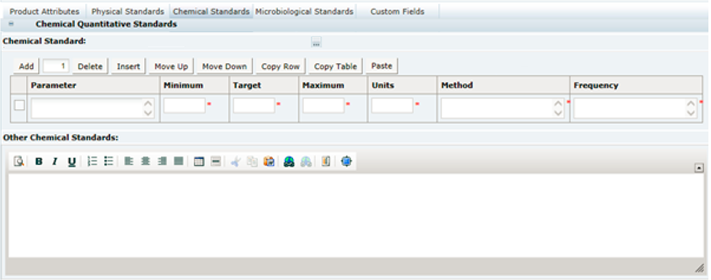
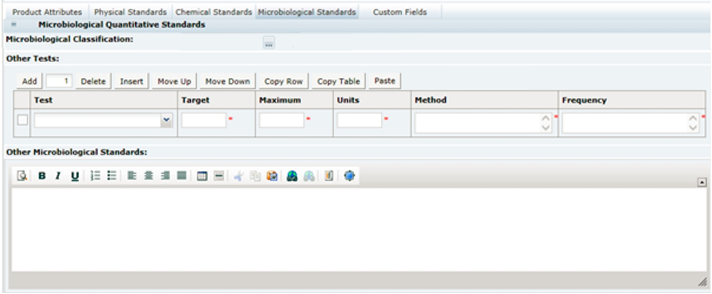
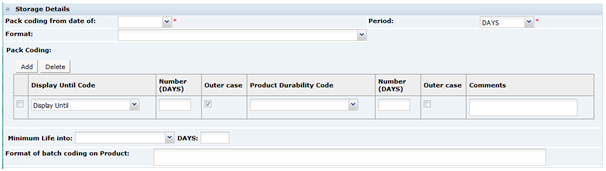
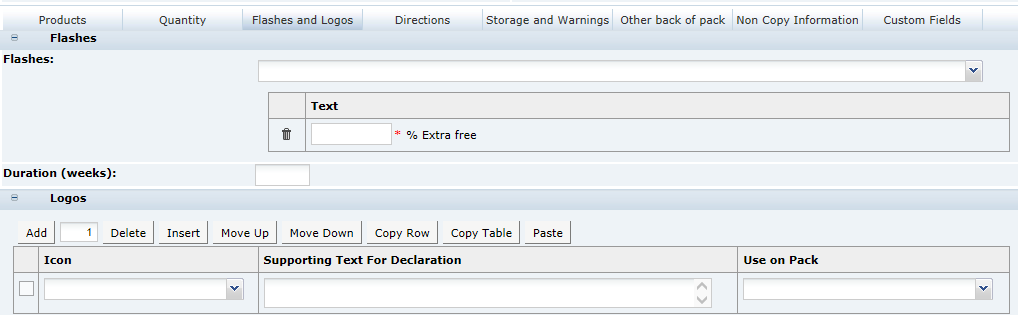
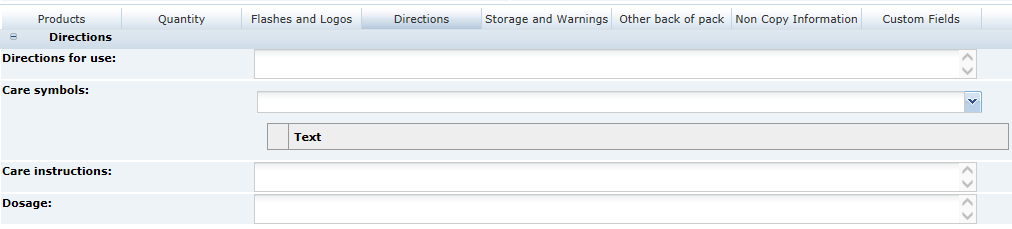
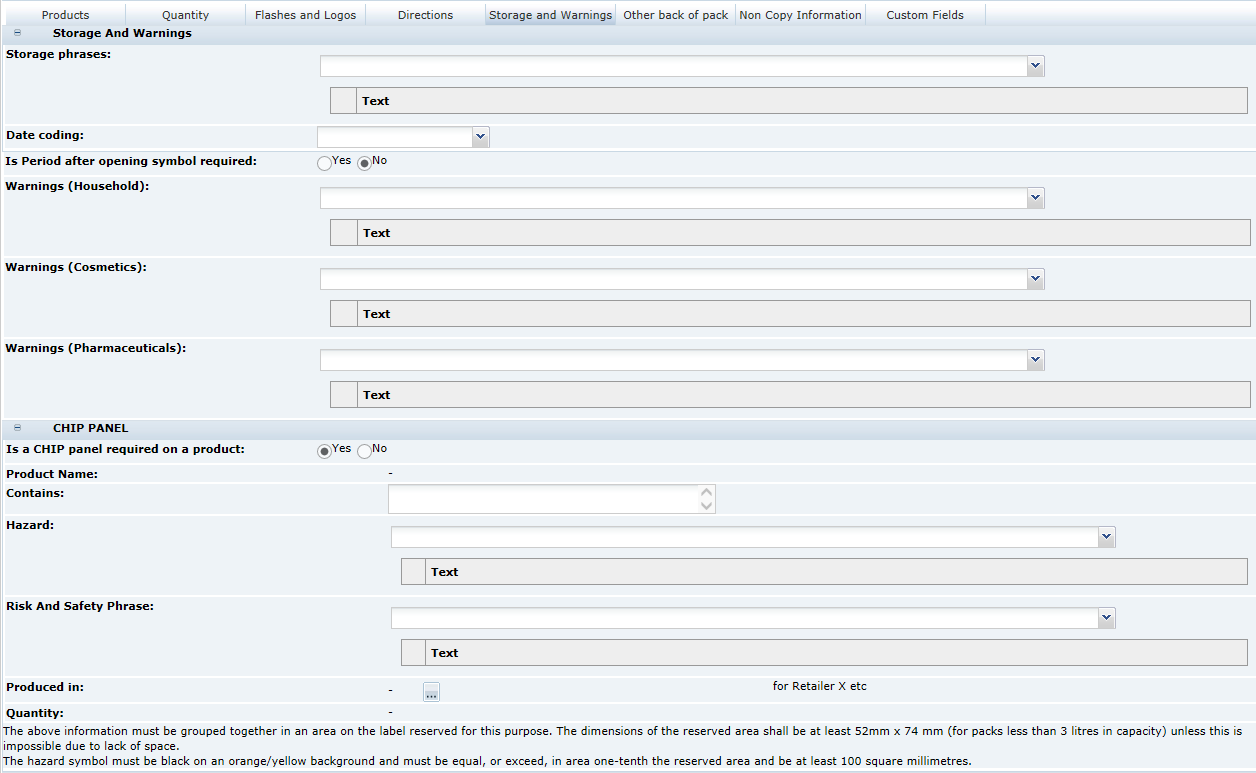
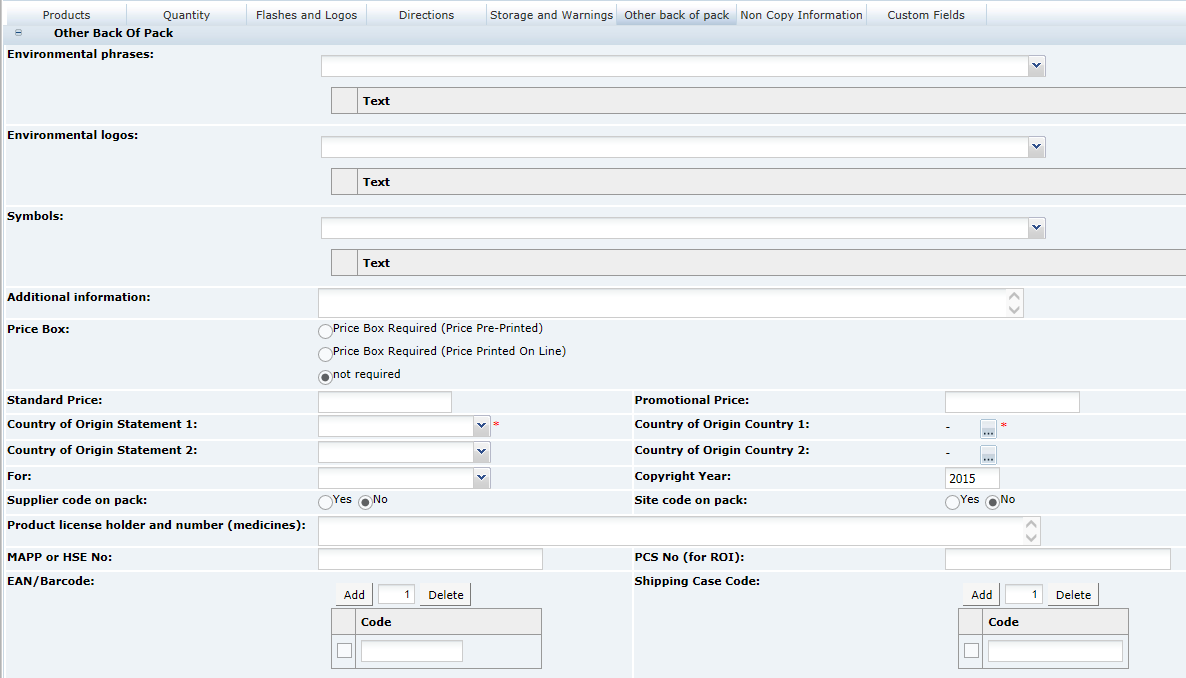
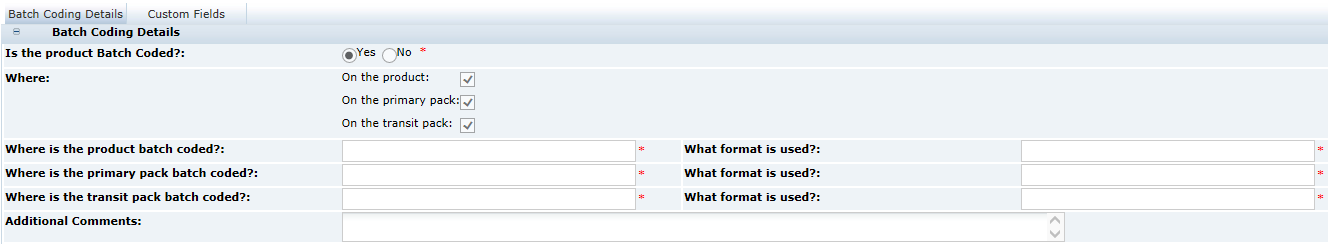
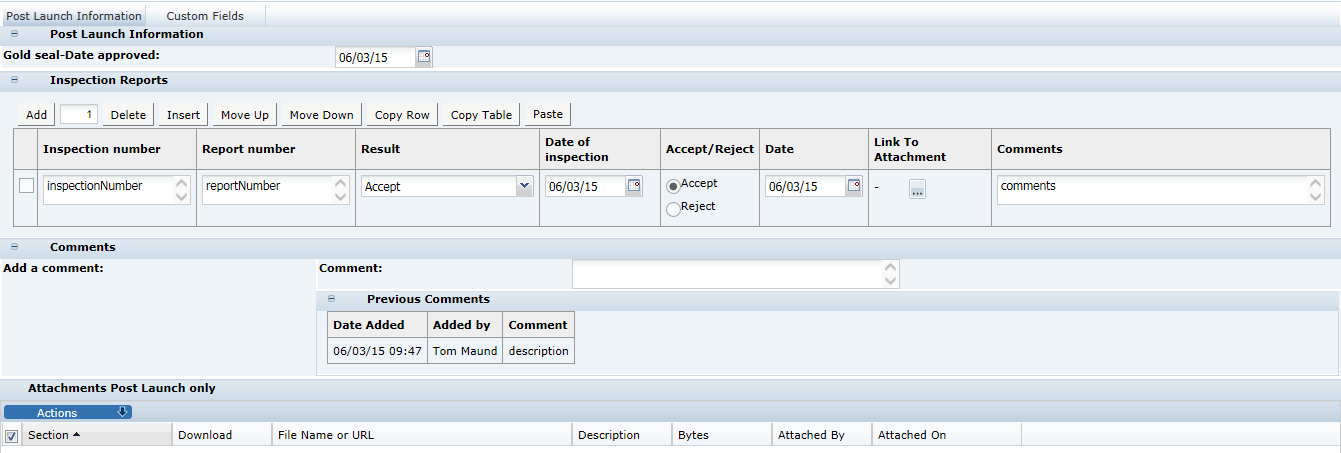
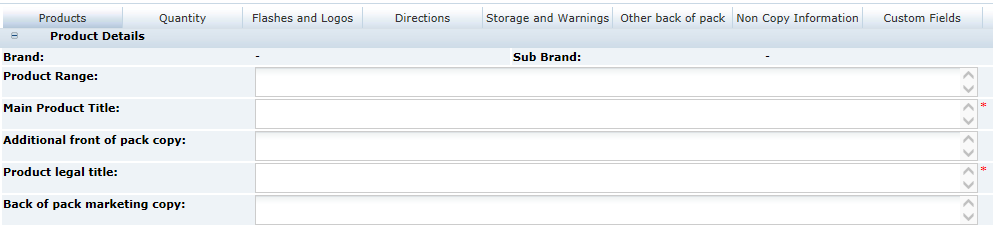
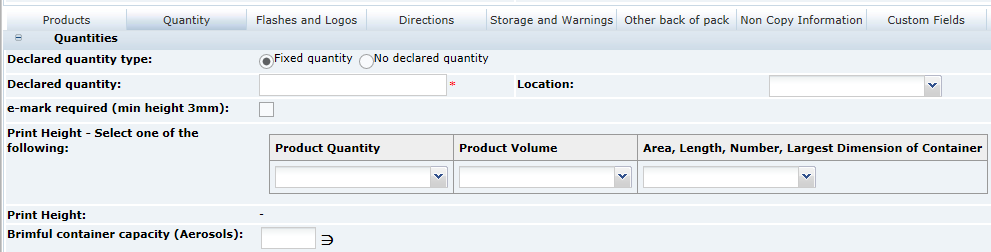
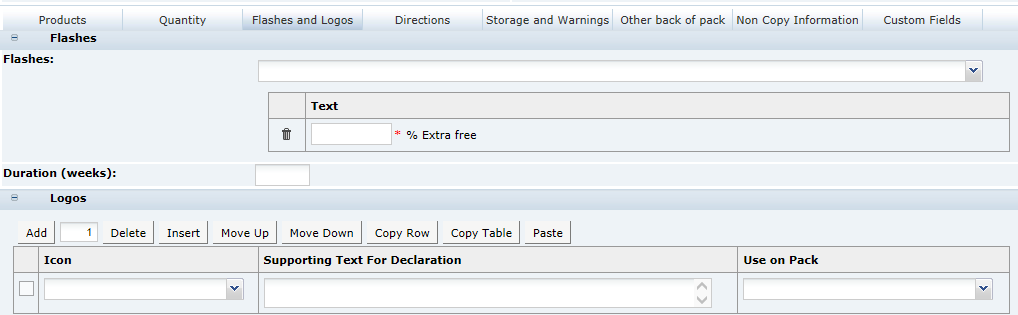
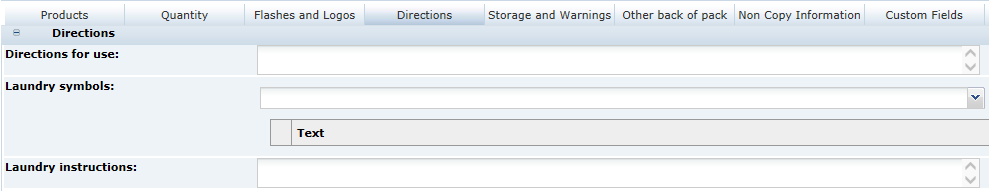
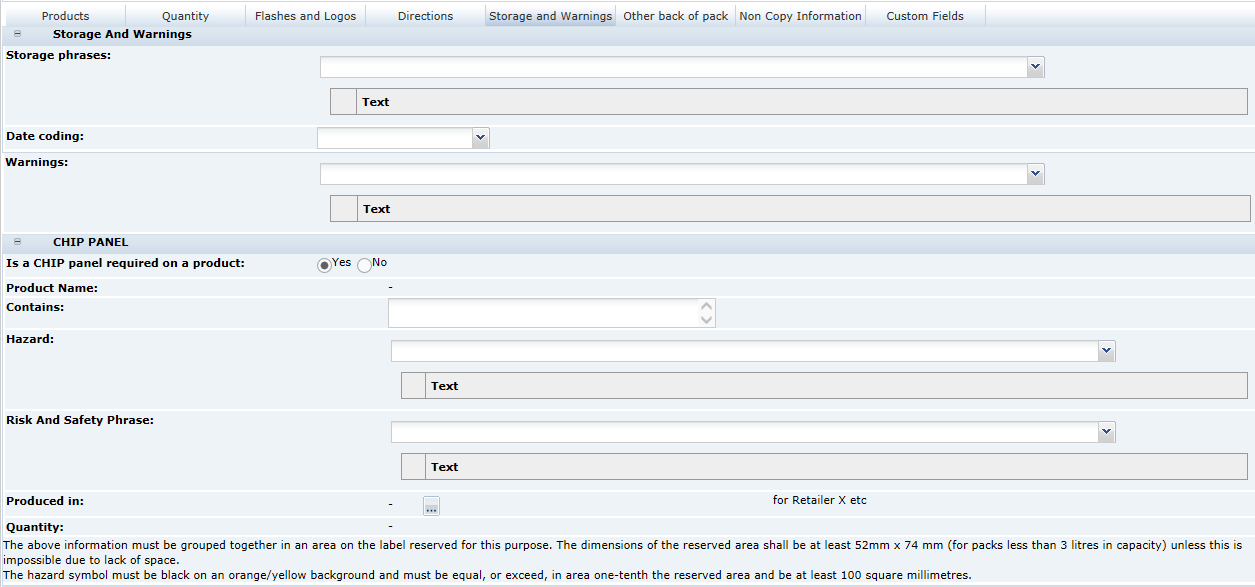
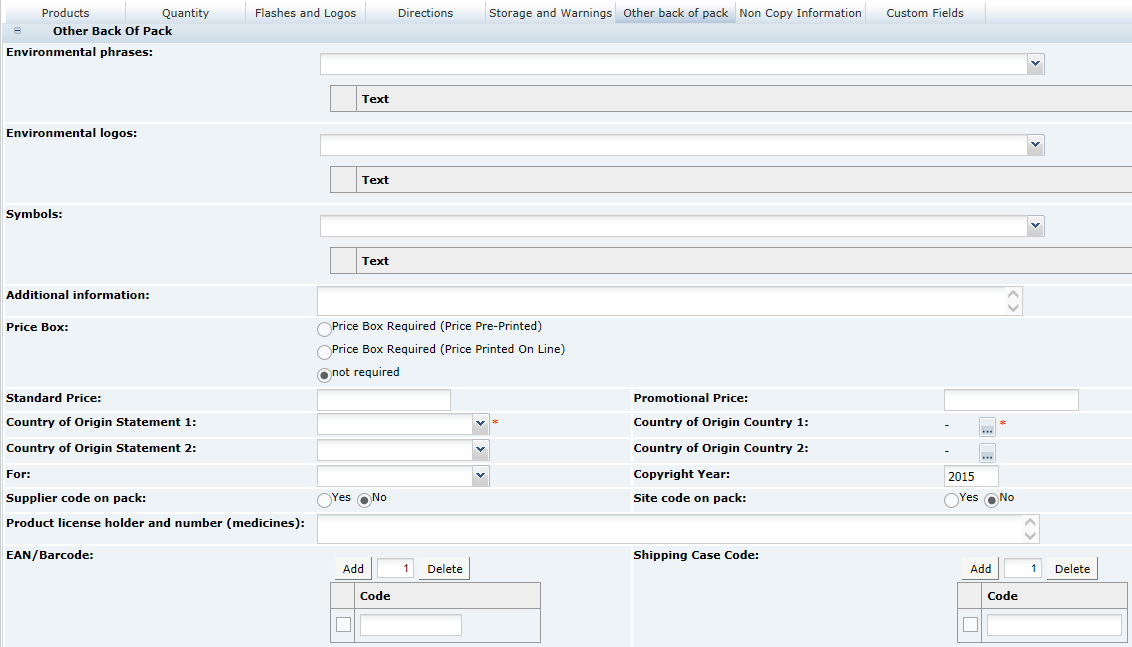
3 Specifications
The Specification captures all technical details about a product, its packaging, processing, and labelling requirements.
Oracle Retail Brand Compliance Management Cloud Service enables Specifications to be created and managed for a retailer's own brand products. Retailer users and Suppliers can work collaboratively in the system to enter and review information, and progress the Specification through Pack Copy generation and eventual sign off of technical product information by both the Supplier and the Retailer.
There are different specification types for different types of product. There are specifications for processed or manufactured products and a special type of specification for fresh produce, that is, unprocessed fruit, vegetables, meat and poultry, and so on.
This chapter describes the specifications for processed or manufactured products.
There are five types of specification for different product types:
-
Pre-Packed Food: Packaged processed foods
-
Counter Food: Bulk supplied processed food sold in store across counters
-
Formulated Non Food: Cosmetics, cleaners, and other chemical formulated products
-
Constructed Non Foods: All types of general merchandise non-foods, such as, toys, cookware, electric items, and so on
-
Beers, Wines, and Spirts: Beers, wines, spirits, and related alcoholic beverages
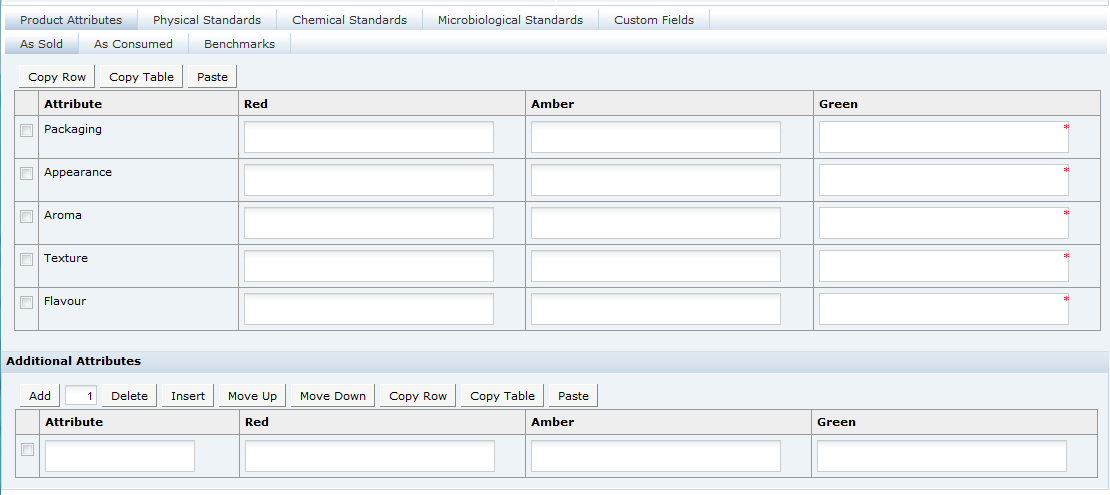
The Specification record is organized into sections, each covering a particular group of information such as the Recipe, Storage, Nutrition, or Packaging.
The default sections which are available depend on the Specification Type: Food, Formulated Non-Food, or Constructed Non-Food. Additional sections may be added, from the same specification type and from a different specification type (Gift Pack for example), but a Specification must always contain at least one of each of its default sections.
As with the Product record, a specification may cover a number of variants for a product, such as, different sizes for the same product. For example, Crisps sold in a 30g and a 200g pack where the crisps are the same in each pack size. Or a specification may cover a product that is made at more than one site from the same Supplier. In these cases, it may be necessary to have certain sections repeated with different details for the different variants. For example, if the product is sold in two types of packaging, a different packaging section is required for each. This may be accommodated in the one specification by adding an additional packaging section and then assigning each of the two to a different packaging variant. This facility to have multiple sections applies to any section except the first, Main Details, section.
Sometimes referred to as a Variety pack, this is a product that is sold as a single packaged item (or SKU), but within the product, there are a number of separately packaged components. Each component may or may not have its own labelling. For these, a special type of specification is created where each of the components has its own set of sections contained within the one specification. A product may have food and non-food components. A specification can be created that combines the sections required for each.
Workflow and Statuses
A specification record has a workflow cycle, during which users may have restricted access or have restricted actions they can take. Data fields become locked at different stages of the workflow. The record has different statuses at each stage of the workflow.
Table 3-1 provides a summary of the different statuses.
Table 3-1 Specification Record Status
| Status | Description |
|---|---|
|
Supplier Draft |
When a specification is created by a Supplier, it has the status of Supplier Draft. The specification can only be seen by the Supplier users with the appropriate permissions. When a Supplier is ready to share the specification with the Retailer, it is moved to Collaborative Draft. Retailer users with the Supplier Draft Specification Visibility authority profile also have access to specifications at this status. They may view, edit, and change the status of the specification per the Supplier user. |
|
Retailer Draft |
When a specification is created by a Retailer, it has the status of Retailer Draft. The specification can only be seen by the Retailer users with the appropriate permissions. When a Retailer is ready to share the specification with the Supplier, it is moved to Collaborative Draft. |
|
Collaborative Draft |
At this status, the specification may be edited by both the Retailer and the Supplier (but not at the same time), so that the specification can be worked on collaboratively. Once the specification is at a suitable stage of completion, the Pack Copy may be issued by the Retailer. This is a document that contains all the legal copy for the label design that is included in the various specification sections. The Pack Copy is issued to an artwork design company through email. |
|
Gate Step |
This step is an optional step, depending on the Retailer's own procedures. The option is set when Oracle Retail Brand Compliance Management Cloud Service is first set up for the Retailer. When this option is used, the specification is forwarded to this step from Collaborative Draft. This stage of the workflow may be used, for example, when the legal copy requires a separate sign off by a Retailer function other than the Technologist prior to the issuance of the Pack Copy. |
|
Pack Copy Sent |
This is the status after the Pack Copy document has been issued. At this stage, any parts of the specification that specifically apply to the legal labelling copy are locked, so that changes cannot be made to the details that have already been issued to the artwork designer. |
|
Ready For Authorization |
A specification may be set to this status by a Retailer user. At this status, all the data fields are locked, ready for the Supplier to authorize or sign off the specification. |
|
Supplier Authorized |
Once the Supplier has authorized the specification, it has this status. The specification is then ready to be signed off by the Retailer. All fields are locked at this status. |
|
Active |
This is the status of the specification once it is signed off by both the Supplier and Retailer. The specification is locked and cannot be returned back to a previous status. |
|
Off-range |
If a product is temporarily taken off sale, for example, it is only a seasonal product, the specification may be set to Off-range to signify that it is not currently being sold, but it may be sold to the same specification in the future. It may be returned to Active when the product is put back on sale. |
|
Delisted |
If the product is removed from sale permanently, the specification is set to this status. It may be returned to Active if the product is put back on sale at a later date. |
|
Superseded |
When a specification is required to be updated, such as, a change to the recipe, new packaging, new label design, and so on, a new version of the Active specification is created. When this new version is moved through the workflow to Active, the original specification is set to active. |
During the workflow of the drafting and approving of a specification, the record may be moved back and forth through these statuses as required, sometimes skipping statuses if they are not required. The system guides the user through the process by only making the different change of status options available that are appropriate to the user at the current status.
At each of the changes of statuses, the system may be configured to send alerting email to the responsible users to inform them of the status change. Additionally, the users responsible for the specification at the Retailer and Supplier may be made aware of the status of their specifications through the Task App that sits on the home page of the system.
Viewing and Creating Specifications
Processed or manufactured product Specifications are listed by selecting the Specifications option under the Product menu.
A list of specifications opens in a new tab. The columns in the list have the following information:
-
Specification Name
-
Specification Number
-
Version
-
Quantity
-
Product Number
-
Technologist
-
Supplier Name
-
Site Name
-
Sub Brand
-
Status
Double clicking on an entry opens the specification in a new browser window.
A new specification may be created from this list view of specifications. The specification is then linked to the appropriate Product Record.
Alternatively, and the preferred method, is to create the specification from within the Product Record that should have been created prior to the specification. For more information, see Chapter 2.
When creating a new specification from the Product Record, options are provided to select the country's legislation that the product is complying with and whether to create a new blank specification or copy an existing specification. There is also an option to create a Multipack specification which will take the user through a wizard to define the individual components and the section requirements.
Pack Copy Files
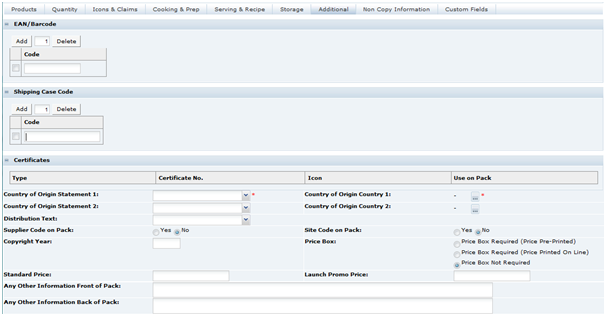
In Product Specifications, the information which will be sent to the design team to be used on a pack is gathered into the Pack Copy File. This information is pulled from the following sections in the Specification:
-
Food:
-
Main Details
-
Recipe and Raw Materials - Declaration
-
Allergy & Dietary Advice - Declaration
-
Nutrition - Declaration
-
Packaging - Recycling Icons
-
Advanced Packaging - Recycling Advice Icons, Other Recycling Icons
-
Other Labelling Copy
-
-
Formulated Non Food:
-
Main Details
-
Formulation and Raw Materials - Declaration
-
Packaging - Recycling Icons
-
Other Labelling Copy
-
-
Produce:
-
Nutrition - Declaration
-
Other Labelling Copy
-
-
Constructed Non Food:
-
Main Details
-
Components
-
Packaging - Recycling Icons
-
Other Labelling Copy
-
-
Beers, Wines, and Spirits:
-
Main Details
-
Product Characterisation and Composition
-
Recipe and Raw Materials - Declaration (Ingredients List and Origin of Meat or Fish are not included)
-
Allergy & Dietary Advice - Declaration
-
Nutrition - Declaration
-
Packaging - Recycling Icons
-
Other Labelling Copy (Country of Origin is not included)
-
Note:
It is possible to override the system-delivered Pack Copy and Counter Ticket layouts with custom formats, which the administrator can design to contain any fields from the product specification.See the Oracle Retail Brand Compliance Management Cloud Service Administration Guide for details.
Previewing Pack Copy
The option to preview the Pack Copy file is available to Retailer and Supplier users when the Specification is at any status. With the Specification in Edit mode, choose Preview Pack Copy from the Action menu.
It is useful to preview the Pack Copy file when reviewing the information which will be sent to the design team, for example to check that the correct Components have been selected to have Pack Copy for multi-pack Specifications.
When using the preview option, the file opens directly on your screen. The Specification will not change status. The preview file is in PDF format, so you cannot edit it, but you can save it locally for future reference if you wish.
Sending Pack Copy
The action to generate and send the Pack Copy file is available only to Retailer users, once the Specification is at Collaborative Draft status. With the Specification in Edit mode, choose Change Status & Exit and then Send Pack Copy from the Action menu.
The option to send the Pack Copy should be used once all the information is complete and ready to be sent to the design team. If any changes are required once the Pack Copy has been sent, the retailer may reject the Specification to an earlier status, allowing edits to be made and the Pack Copy resent.
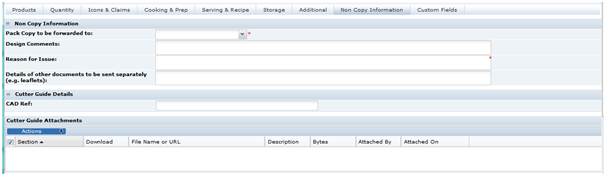
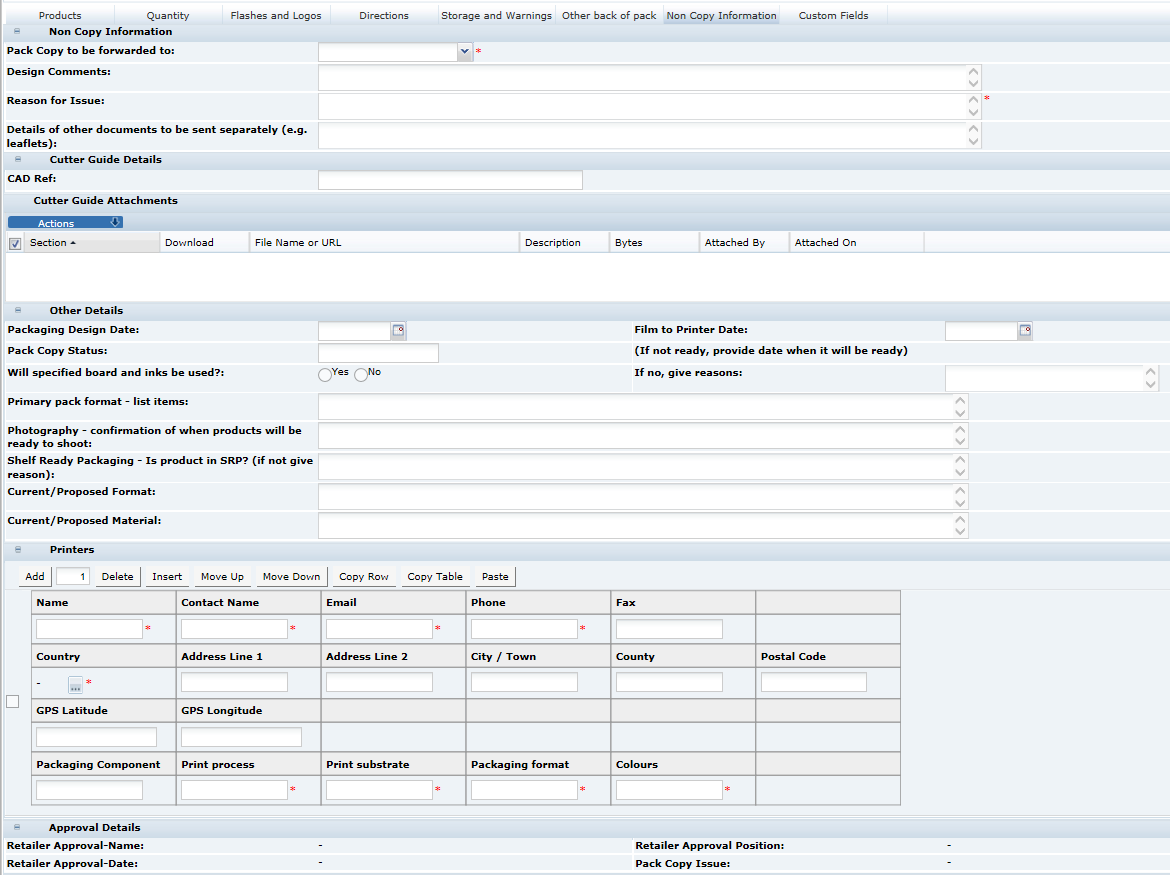
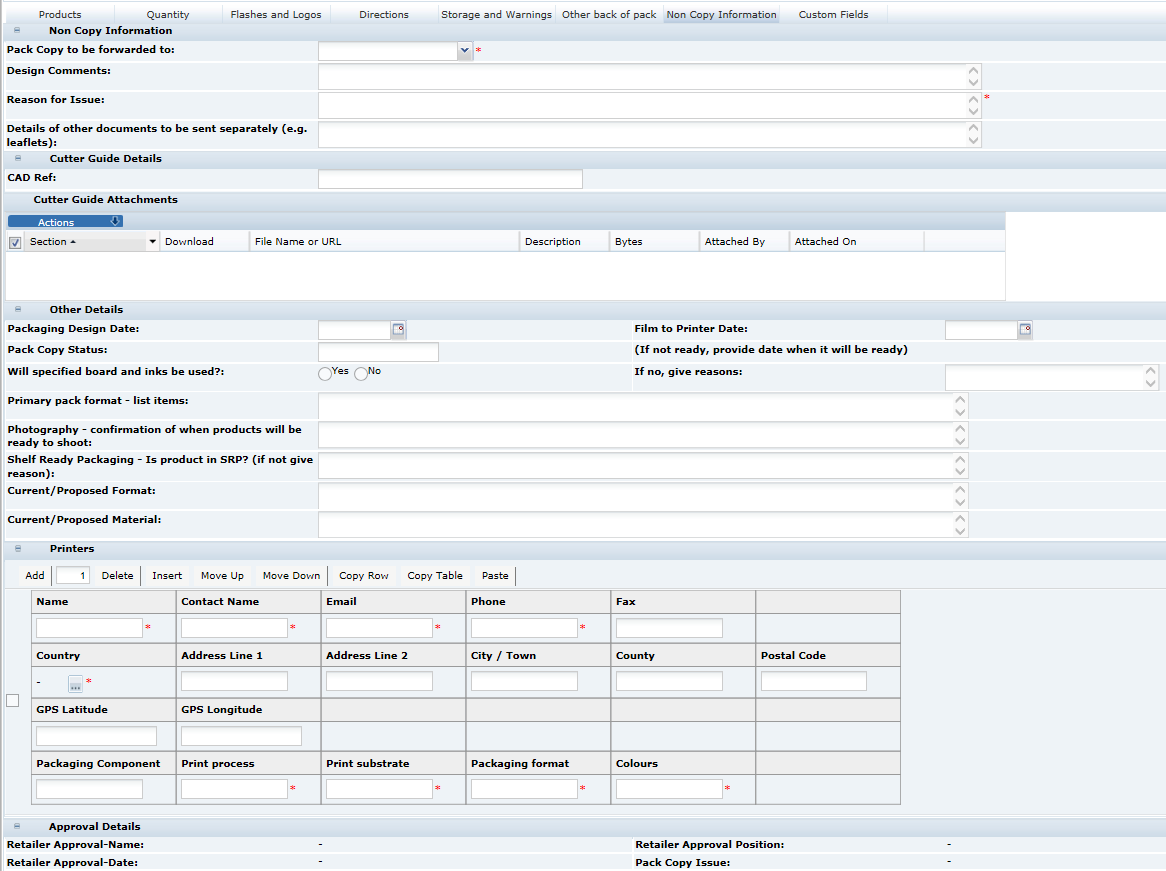
When sending the Pack Copy, the Specification status is updated (to Pack Copy Sent) and the Pack Copy files are sent in email (as either PDF, Microsoft Word, or zipped Microsoft Word files) to the design contact (Pack Copy to be forwarded to specified in the in the Specification's Other Labelling Copy section). The email also includes any Cutter Guide attachments that are present in the Other Labelling Copy section.
In addition, the files are automatically attached to the Product Record for future reference.
The action to generate and send a partial Pack Copy file is available as an alternative where the full information has yet to be captured in the Specification. With the Specification in Edit mode, choose Change Status & Exit and then Send Part Pack Copy from the Action menu. This sends the Pack Copy file as per the Send Pack Copy option, but sets the Specification status to Part Pack Copy Sent. From this status, the Send Pack Copy option can be used once the full information has been captured in the Specification.
Note:
For multi-variant/size or multi-site Specifications where Pack Copy files could be created for each of the variant/size or site combinations, these are collated into a single document for the preview, but will be created as separate files when sending the Pack Copy, since these may each be sent to different design contacts.Multi-Specifications: Choosing what Pack Copy Files to Create
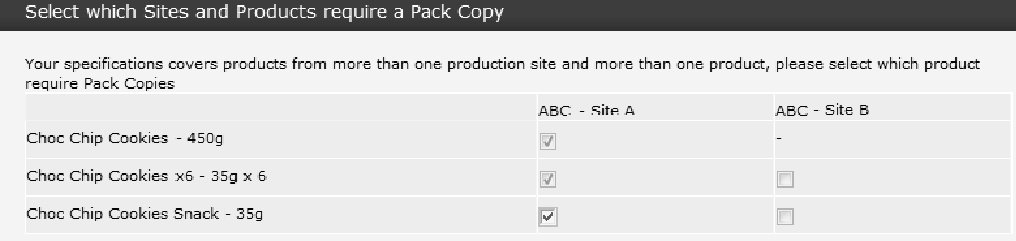
When either previewing or sending a Pack Copy for multi-variant/size, multi-site, multi-pack, and alternative requirement Specification, you must select what Pack Copy files you wish to create.
Multi-Variant/Size and Multi-Site Combinations
If the Specification covers more than one Product, more than one Site, or a combination of Products and Sites, the system prompts you to choose which of the Product or Site combinations you wish to create a Pack Copy for on this occasion. This prompt appears at the time when you preview or send a Pack Copy.
Check the boxes to indicate which Products and Sites require a Pack Copy on this occasion. When sending a Pack Copy, a separate file is created for each selection. In the example above, the Specification covers three Product variants, which are associated with two Sites. It is selected to create Pack Copy files for all three Products, but only for Site A.
-
If there are no differences between the Pack Copy information for each Site (the Sites will be sharing the packaging), you do not necessarily need to create Pack Copy files for every Site as it will be the same.
-
Once you have sent a Pack Copy, you may find that amendments are required to some of the Products but not all of them, therefore, when re-sending a Pack Copy, you may choose only the Products for which amendments are required.
Alternative Requirements
If the Specification contains multiple sections to accommodate alternative requirements for the Product, such as seasonal differences in the ingredients or formulation, you are prompted to choose which one of the alternative sections you wish to apply to the Pack Copy. This prompt appears when you preview or send a Pack Copy.
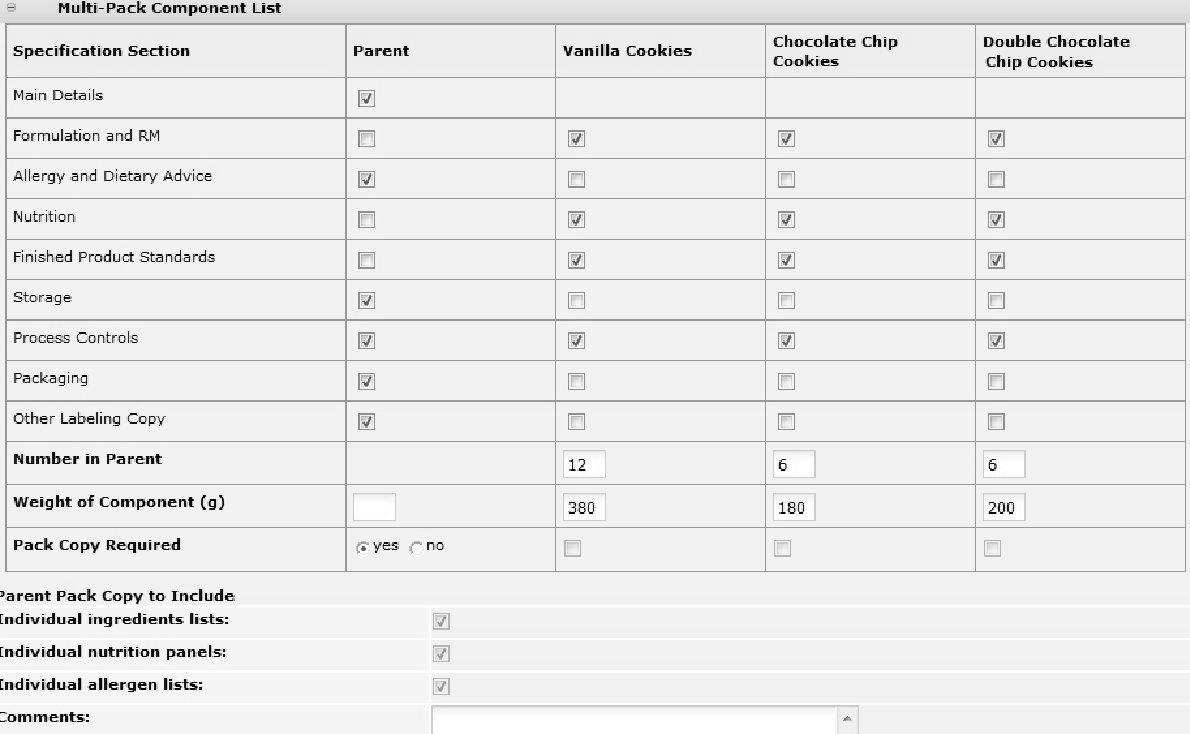
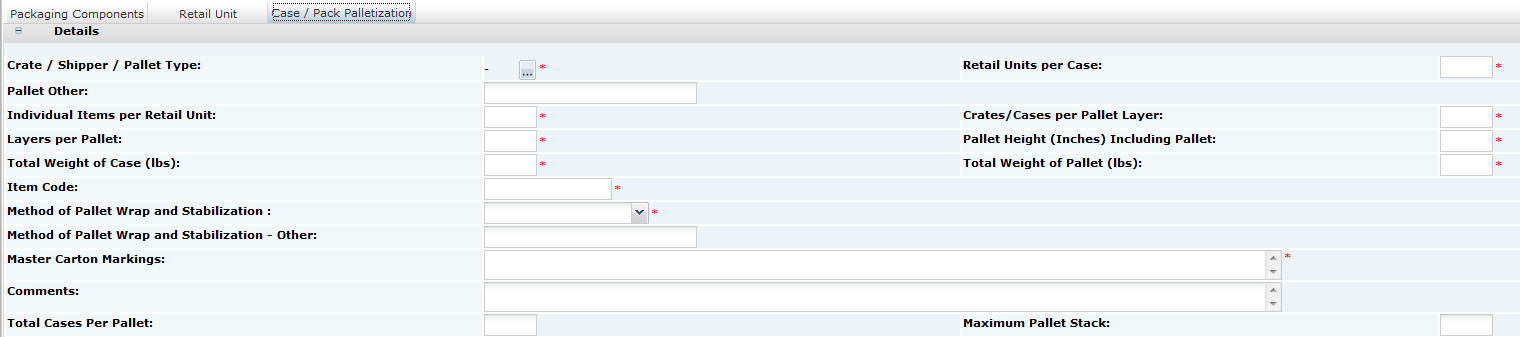
Multi-Pack Specifications
Multi-pack Specifications contain a special Multi-Pack Components List section, which provides a summary of all the components within the Specification and which enables you to confirm which parts of the Product requires Pack Copy information.
The Pack Copy Required details must be completed before you either preview or send the Pack Copy. The options which are selected here depend entirely on the labelling requirements of the particular Product in question:
-
Parent:
Select either yes or no to indicate if a parent (composite) Pack Copy file is required. If the parent Pack Copy file is required, there must be a parent Other Labelling Copy section present in the Specification.
When selecting yes, you may also indicate whether the parent Pack Copy should include the individual Ingredients Lists, Nutrition Panels, and/or Allergen Lists for each Component. These elements will be included with the parent Pack Copy file.
-
Each Component:
Check the box for any Component where a separate Pack Copy file is also required. This would be in the case where any of the Components are separately packaged and labelled. If a Pack Copy file is required for any of the Components, an Other Labelling Copy section must be present for each.
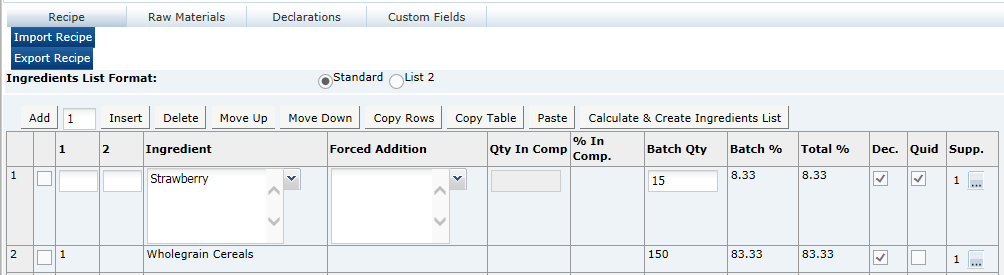
Recipe and Formulation Upload
The Recipe and Raw Materials section in the Food Specification and equivalent Formulation and Raw Materials section in the Formulated Non Food Specification include a facility for the user to import and export the recipe/formulation and associated raw materials using a Microsoft Excel spreadsheet. The facility is typically used to export an existing recipe/formulation to the spreadsheet, either for it to be completed offline before being imported back into the system or for it to be imported into a different Specification. However, it can be used to create a recipe/formulation from scratch.
The import/export options are available to all users that have the ability to edit the Specification (where the feature is enabled in the portal, and the Specification is at a status where it may be edited). The export option can be used when the Specification is at any status. The import option can only be used when the Specification is at a status where the recipe/formulation table is editable, that is, prior to Pack Copy Sent.
If the import/export feature is enabled, the buttons show at the top of the recipe/formulation table:
Exporting a Recipe or Formulation
To export a recipe or formulation:
-
Click Export Recipe or Export Formulation.
-
Select Open. To save to a local location from which to open the spreadsheet, select Save or Save As. The recipe/formulation is opened as a spreadsheet.
Importing a Recipe or Formulation
To import a recipe or formulation:
-
Click Import Recipe or Import Formulation. To locate the spreadsheet file (.xls file) to import, click Browse.
-
Click Ok. A warning is issued that continuing will overwrite the recipe/formulation and raw materials.
-
Click Ok to continue. The contents of the spreadsheet are validated. If no error are encountered, the recipe or formulation and raw materials tables are updated from the spreadsheet.
-
To validate the recipe or formulation and generate the ingredients list, click Calculate & Create Ingredients List.
Spreadsheet Layout
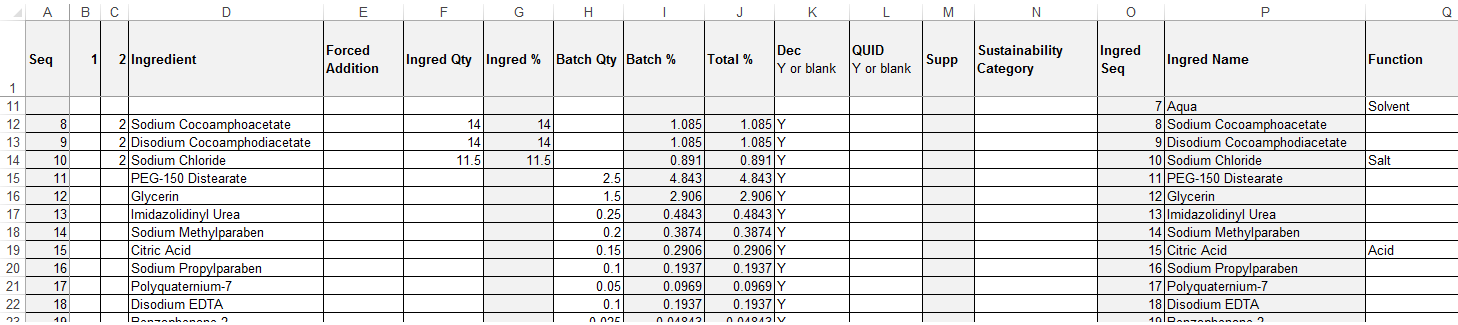
The spreadsheet represents the Specification's recipe or formulation and associated raw materials as a single extended table. The columns of the raw materials table are shown to the right of the recipe/formulation table columns.
If an ingredient's raw materials are supplied by more than one supplier, it is shown as consecutive rows for the raw materials, but with the recipe/formulation details just on the first. The greyed-out columns indicate the columns that are omitted if the file is imported back into the system. These columns are exported for reference purposes only.
The file format must be .xls (Microsoft Excel spreadsheet workbook). The first row contains the column headings which correspond to those in the Specification. Translations are exported where used.
Any rows that contain no values in any column are ignored, however, any rows or columns that contain values but have been hidden in Excel will still be processed. It is possible to just populate the Recipe/Formulation tables (that is, without any raw materials details), however, it is not possible to just populate the Raw Material table.
Table 3-2 describes the columns of the spreadsheet and the main validation rules.
Table 3-2 Upload Spreadsheet Columns
| Column | Description and Validation |
|---|---|
|
Recipe/Formulation Table |
|
|
Sequence |
A unique sequence number to identify the row within the recipe/formulation. It is used to link rows in the raw materials file to their ingredient. Not imported. |
|
1 |
Used to define compounds and their ingredients. Not validated, but should follow the convention for linking ingredients to compounds, and so on. |
|
2 |
Used to define compounds and their ingredients. Not validated, but should follow the convention for linking ingredients to compounds, and so on. |
|
Ingredient |
Name of the ingredient. Not validated, but an attempt is made to match the ingredient to the ingredients glossary (first by ingredient name, then by alias) in a similar way to how tabbing out of the field when manually editing the recipe attempts to auto-select a value. |
|
Forced Addition |
Name of a forced addition, such as, and additive or processing aid. No validation applied, but an attempt is made to match the value to the glossary (based on the Specification's pack copy language). |
|
Qty In Comp |
Must be numeric. |
|
% In Comp. |
Not imported. |
|
Batch Qty |
Must be numeric. |
|
Batch % |
Not imported. |
|
Total % |
Not imported. |
|
Dec. |
Whether the ingredients are declared or not. Valid options are Y (checked) or blank (unchecked). Any other value is set to blank. |
|
Quid |
Only used in recipes. Indicates if the ingredient is subject to QUID. Valid options are Y (checked) or blank (unchecked). Any other value is set to blank. |
|
Supp |
The number of suppliers of the ingredient. Not imported. |
|
Sustainability Category |
If present, must exist in the Sustainability Categories glossary. |
|
Raw Materials Table |
|
|
Sequence |
Not imported. Included for reference only. Provides reference to the associated row in the recipe table when the export option is used. |
|
Ingredient |
Not imported. Included for reference only. Provides reference to the associated row in the recipe table when the export option is used. |
|
Function |
Only used in formulations. If present, must exist in the Functions glossary. |
|
Assurance Standard |
Only used in recipes. If present, must exist in the Assurance Standards glossary. Can contain multiple values, separated by a tilde. |
|
Trade Name |
Only used in formulations. |
|
Grade/Specification |
Mandatory if Raw Materials Info column is Yes. |
|
Supplier |
Mandatory if Raw Materials Info column is Yes. |
|
Site Location |
Only used in recipes. |
|
Year of Last Animal Test |
Only used in formulations. If present, must exist in the Year of Last Animal Test glossary. |
|
Reason For Animal Test |
Only used in formulations. If present, must exist in the Reason for Animal Test glossary. |
|
Country where Processed |
Only used in recipes. Mandatory if Raw Materials Info column is Yes. If present, must exist in the Countries glossary. Can contain multiple values, separated by a tilde. |
|
Country Of Origin |
Mandatory if Raw Materials Info column is Yes. If present, must exist in the Countries glossary. Can contain multiple values, separated by a tilde. |
|
Raw Materials Info |
Whether raw materials information is mandatory for the ingredient. Valid options are Yes and No. Any other value will default to Yes. |
|
Breed/Latin Name/Variety |
If present, must exist in the Breed/Latin Name/Variety glossary. Can contain multiple values, separated by a tilde. |
|
Source |
If present, must exist in the Source glossary. Can contain multiple values, separated by a tilde. |
|
Catch Method |
If present, must exist in the Catch Method glossary. Can contain multiple values, separated by a tilde. |
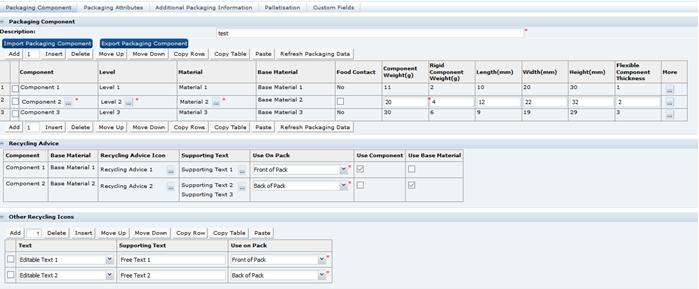
Advanced Packaging Import and Export
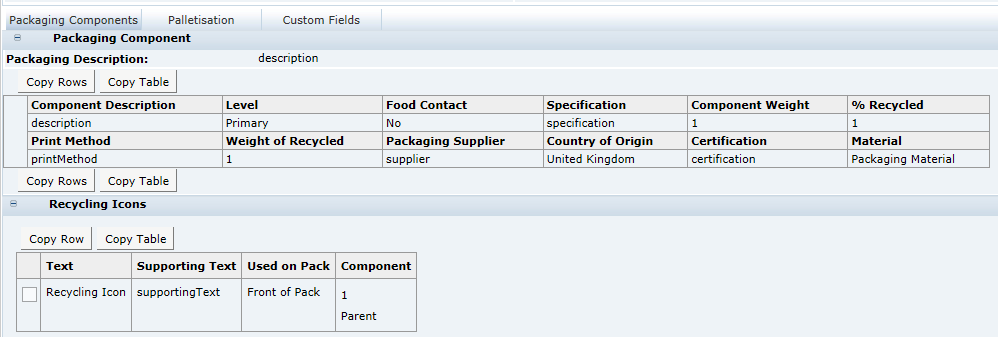
The Advanced Packaging section in each of the available specification types includes a facility for the user to import and export Advanced Packaging data using a Microsoft Excel spreadsheet. This facility is similar to other import and export functionality available within specifications.
The availability of the functionality is configurable and, if enabled, the Import and Export buttons will appear in the Advanced Packaging section while in edit mode. The buttons will appear above the Packaging Component table. If disabled, the Import and Export buttons will not appear in any specification types. Access to the parameter is per the relevant existing Authority Profiles for System Parameters.
Exporting Advanced Packaging Data
To export advanced packaging data:
-
Click Export Packaging Component.
-
Select Open. To save to a local location from which to open the spreadsheet, select Save or Save As.
Importing Advanced Packaging Data
To import advanced packaging data:
-
Click Import Packaging Component. To locate the spreadsheet file (.xls file) to import, click Browse.
-
Click OK. A warning is issued that continuing will overwrite the Advanced Packaging data.
-
Click OK to continue. The contents of the spreadsheet will be validated for:
-
Correct Specification Type
-
Missing mandatory fields
If no errors are encountered, the advanced packaging tables are updated from the spreadsheet. Any invalid glossary entries or other invalid data will not be populated.
-
-
A confirmation prompt will confirm the import has completed.
Spreadsheet Layout
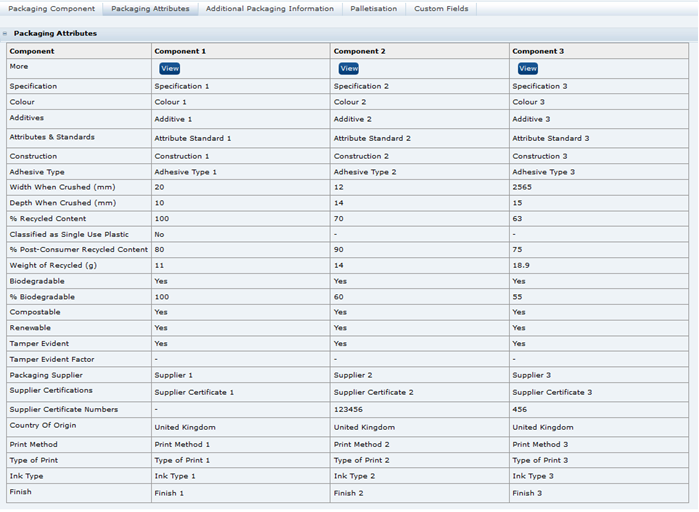
The exported spreadsheet includes a number of worksheets. The first worksheet is the Advanced Packaging Component Details and includes all the fields from the Packaging Component and Packaging Attributes tables. The remaining tabs list glossary codes for each of the component details and attributes that use glossaries. The codes are provided and identified for all specification types.
Food Specification
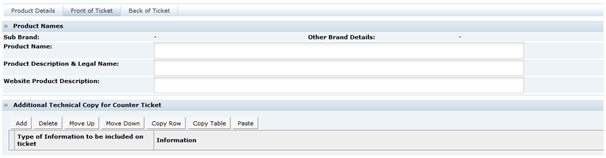
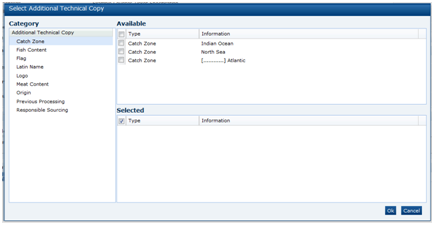
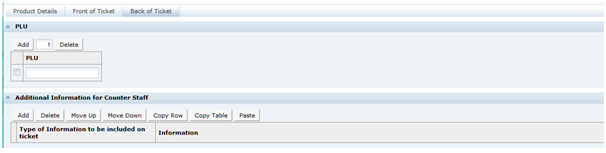
There are two types of Food specification, the Pre-Packed Food and Counter Food specifications. These differ by only one section. Both contain the following sections:
The Pre-Packed Food specification has an additional section called Other Labelling Copy. The Counter Food specification has an additional section called Counter Ticket. Each section has a common header at the top with the basic information about the specification.
Table 3-3 describes the fields in the header.
Table 3-3 Specification Header Fields
| Field | Description |
|---|---|
|
Specification Name |
This is copied from the Product Record when the specification is created. It is to be sufficiently precise to enable the Product to be identified by both the Supplier and other Retailer users, and to distinguish it from other similarly-named products. |
|
Specification Number |
An automatic sequential number set by the system. |
|
Version |
The specification is version controlled. This is an automated number set by the system to distinguish this version from previous versions. |
|
Status |
The status the record is automatically set to after a user has set the specification to the next required status in the workflow. |
|
Specification Type |
The type of specification record that was selected. |
|
Supplier Name |
The company or agent supplying this product. |
|
Supplier Code |
The code of the Supplier within the Oracle Retail Brand Compliance Management Cloud Service system. |
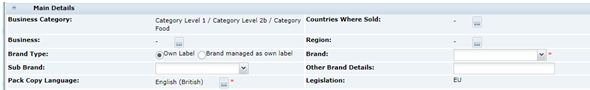
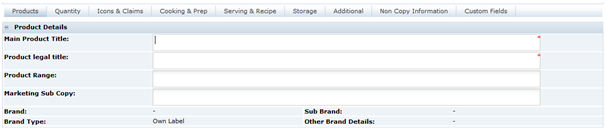
Main Details Section
The Main Details section covers the primary details about the product, such as the manufacturing Supplier and Sites, Brand details and Key Dates, and the specification history.
In common with other sections of the specification, the Main Details is split into sub-sections referred to as field sets:
Main Details
Table 3-4 describes the fields.
Table 3-4 Food Specification Main Details Field Set
| Field | Description |
|---|---|
|
Business Category |
This is copied across from the Product Record. It is the commercial category in which the product is sold. |
|
Countries Where Sold |
A list of countries where the product is approved to be sold. |
|
Business |
Which, if any, the different sub-businesses where the product is approved to be sold, such as, in store and on-line. |
|
Region |
Any regions that the product may be restricted to in its sale. |
|
Brand Type |
Whether the product is a retailer's own brand or whether it is an manufacturer's brand that is treated as though Own Brand. |
|
Brand |
Where the Brand Type was set to Own Label, the specific retailer's brand name. For some retailers, there may only be the one option in the selector.If Brand Managed as Own Label was selected in the Brand Type, this field is free text and contains the manufacturer's brand name as it appears on the label. |
|
Sub Brand |
For retailers that have various sub brands for their own label. |
|
Other Brand Details |
This field is for any other brand naming that is required on the label, not covered by the previous two fields. |
|
Pack Copy Language |
The language to be used on the label. This defaults to the language of the user that created the specification. If changed, the system requires the user to save and close the specification and then reopen it to continue. This is to allow the system to update the specification accordingly. |
|
Legislation |
This is the legislation chosen when the specification was created. This cannot be changed. |
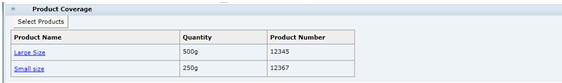
Product Coverage
This shows a copy of the Products Covered table that was completed in the Product Record. If required, while at draft, it is possible to reselect a different Product record to link the product to by clicking Select Products.
The portal may be configured to include additional Alt. Product Number and Division fields in the Product Coverage table. The alternative product number can be used to hold the product's identifier such as a GTIN code; Division is selected from a glossary of categorizations. If used, the new fields are synchronized between the Product Record and Specification as per the existing Product Coverage fields.
If the Alt. Product No. and Division fields are used, the portal may be further configured to use the fields to control the updating of the Retailer Product Number. This would typically be used where the Retailer Product Number holds the product's identifier from an external system, such as an Article Number. If this updating is enabled, when a Product Record is saved, any rows in associated Specifications that have the same Alt. Product Number/GTIN and Division values have the Retailer Product Number/Article Number replaced with the corresponding value from the Product Record. For Produce specifications, the update is from the Specification to the Product Record, on supplier acceptance.
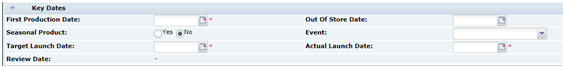
Key Dates
Table 3-5 describes the fields.
Table 3-5 Food Specification Key Dates Field Set
| Field | Description |
|---|---|
|
First Production Date |
The date of the planned first production of the product. |
|
Out of Store Date |
This field is completed using a dialog box when the specification is superseded by another version or if the specification is set to the status of Off-range or Delisted. This field represents the date when the product, as specified in this version, is expected to no longer be sold in stores. If the Seasonal Product field below is set to Yes, a date is filled in here to say when the seasonal product is to no longer to be sold in stores. |
|
Seasonal Product |
Out of Store Action: If Yes is selected, the Out of Store Date must be completed. In addition, the relevant option must be selected to indicate whether the Specification should be De-listed (for once-off events), or set to Off Range (for cyclical events) when the Out of Store Date passes. |
|
Event |
Select an event which the product is marketed or branded towards, if applicable. |
|
Target Launch Date |
Represents the expected in-store date of the product. |
|
Actual Launch Date |
Represents the actual in-store date of the product; set before the Specification is made Active. |
|
Review Date |
Cannot be edited in the Specification itself, but is set at the time the Specification is made Active through the menu which appears as part of the Make Active process. This represents a future date when the Specification must be reviewed. The Specification will be flagged as Due for Review once this date passes, and will appear in the Task Manager of the Retailers Contacts named in the Specification and the Supplier's Specification Admin contacts. Upon review, a New Version of the Specification may be created to submit updated information, or the Retailer may extend the Review Date for a further period. |
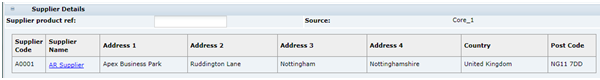
Supplier Details
Table 3-6 describes the fields.
Table 3-6 Food Specification Supplier Details Field Set
| Field | Description |
|---|---|
|
Supplier Product Ref |
Free text field, allowing the Supplier to enter a reference which is meaningful to the Supplier's company in terms of identification, such as, Factory Recipe Numbers or product identification codes. |
|
Source |
Automatically completed by the system, shows the name of the Retailer's system or another source of the data, if applicable. |
|
Supplier Code, Name, and Address |
Details of the Supplier that supplies the product to the Retailer. Pulled from the linked Product Record. The Supplier Name provides a link to open the Supplier Record in the main window. |
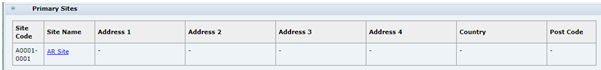
Primary Sites
Table 3-7 describes the fields.
Table 3-7 Food Specification Primary Sites Field Set
| Field | Description |
|---|---|
|
Site Code, Name, and Address |
Details of the Primary Sites which manufacture this Product for the Retailer. Pulled from the linked Product Record. The Primary Sites are those which have direct responsibility for the product. The Site Name provides a link to open the Site record. |
Secondary Sites
Table 3-8 describes the fields.
Table 3-8 Food Specification Secondary Sites Field Set
| Field | Description |
|---|---|
|
Site Code, Name, and Address |
Any sites, other than the primary site, involved in the intermediate production of this product. Note: The primary site is usually considered to be the last site at which processing quality control is applied. There are two options to complete: If the secondary site details are set up on the system under the respective Supplier, the details may be copied across by clicking Select Site. If not, clicking Add provides free text fields to enter the details. |
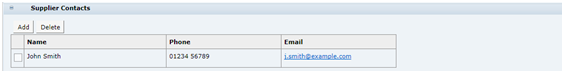
Supplier Contacts
Table 3-9 describes the fields.
Table 3-9 Food Specification Supplier Contacts Field Set
| Field | Description |
|---|---|
|
Name, Phone, and Email |
Name, Phone, and Email In a new Specification, automatically populated with any Contacts with the Specification Admin role at the associated Supplier and Sites, at the time the Specification is created. Click Add to select additional Contacts. Click Delete to remove Contacts. Remove any Contacts who are not directly responsible for this Specification or who do not need to receive notifications about the Specification. |
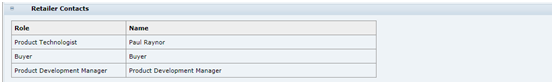
Retailer Contacts
Table 3-10 describes the fields.
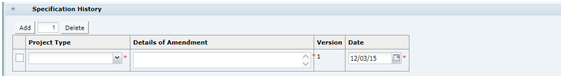
Specification History
The Specification History is blank in a new Specification, including when a copy is made from an existing Specification. Use Add and Delete to make entries to the table each time updates to the Specification are made. This will assist other users when they review the Specification.
It is good practice to update the table when a specification is being updated to have more visibility to changes being made (in addition to Change History). The specification retains the same version until after Active and a New Version is created.
In a New Version of a Specification, the Specification History from the previous version is copied and locked. Start adding new entries to the table to record updates to this version.
Table 3-11 describes the fields.
Table 3-11 Food Specification History Field Set
| Field | Description |
|---|---|
|
Project Type |
Represents the reason for work being carried out on the Specification. |
|
Details of Amendment |
Provide a description of the updates made to the Specification on this occasion, for example: Amendments to formulation to reduce salt content, updates to Allergen and Nutrition information in line with formulation changes. |
|
Version |
The current Specification version, set automatically by the system. |
|
Date |
The date the current updates are completed. |
Final Approval
Table 3-12 describes the fields.
Table 3-12 Food Specification Final Approval Field Set
| Field | Description |
|---|---|
|
Supplier Approved Name, Position, and Date Retailer Approved Name, Position, and Date |
Completed using dialog boxes by the Retailer and Supplier when the Specification is progressed through the workflow. Represents sign off of the Specification by the Supplier and the Retailer. Supplier Approver Details: When setting the Specification to Supplier Authorised. Retailer Approver Details: When setting the Specification to Active. |
Declaration
This is a declaration of conformity to which the Supplier is agreeing to during sign off when setting the specification to Supplier Authorised.
Attachments
Documents relevant to the product specification may be attached in any section or within the Attachments section itself. To show the attachments or to add or delete an attachment, click Show Attachments. This sub-section is available in all sections of the specification.
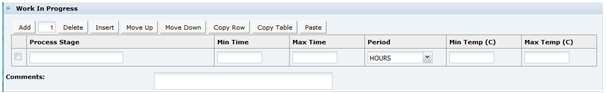
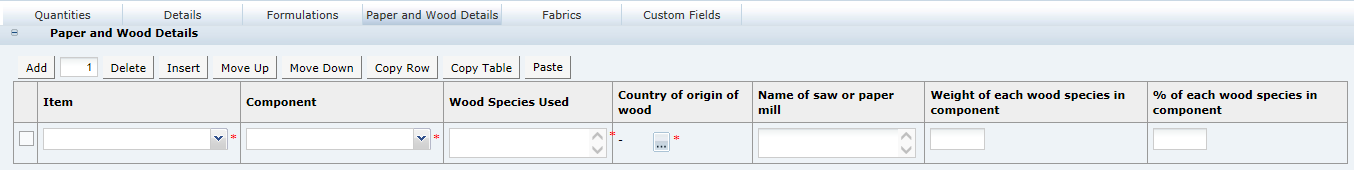
Recipe and Raw Materials Section
This section has the following tabs:
-
Sustainability Tab (if Sustainability is enabled)
-
Material Transparency Tab (if Material Transparency is enabled)
Recipe Tab
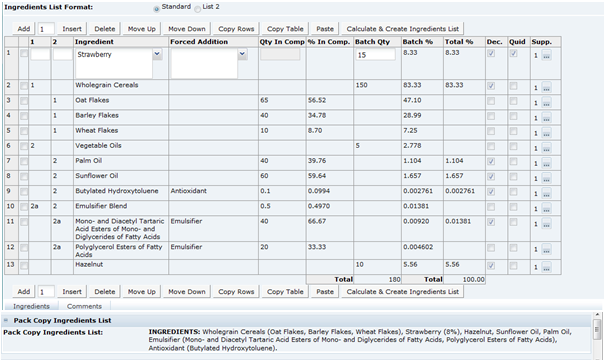
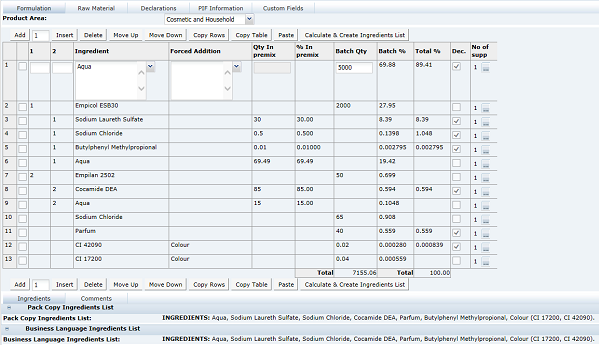
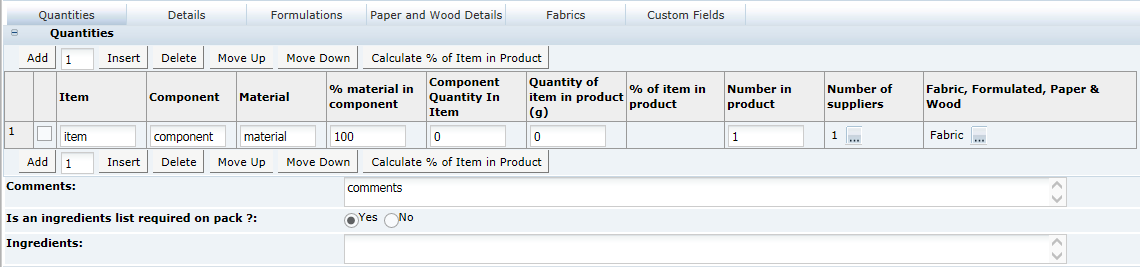
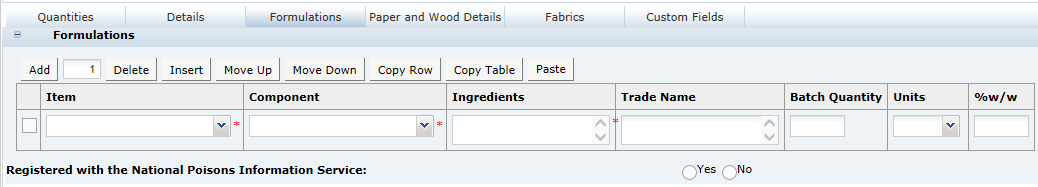
This tab provides details of the Recipe. All ingredients, compound ingredients (or sub-recipes) and component ingredients are listed with quantities and whether they are to be declared.
When working in a new Specification, use Add, Insert, and Delete to add sufficient rows to the table for all of the ingredients in the formulation.
Table 3-13 describes the fields.
Table 3-13 Food Specification Recipe Page
| Field | Description |
|---|---|
|
Ingredient List Format |
Standard and List 2 are the options. This controls the layout of the ingredients list that is created by the system when clicking Calculate and Create Ingredient list. Standard creates an ingredient list with the components of compounds in brackets after the compound name. List 2 separates the compound components onto another line. |
|
1 |
The column with the title 1 is used to indicate Compound Ingredients. Use 1, 2, 3, and so on, to indicate the different compounds in the recipe. If this Ingredient is not a Compound Ingredient, leave the column blank. Refer to Single Ingredients, Compounds and Components beneath this table. Alternatively, a number sign (#) may be entered into this column in order to insert a Comment row or blank row into the formulation, which is not to appear on the label; this helps make reviewing a lengthy recipe easier. |
|
2 |
The column with the title 2 is used to indicate the Components of a Compound with the same number as given in column 1. If this Ingredient is not a Component of a compound, leave the column blank. Refer to Single Ingredients, Compounds and Components beneath this table. |
|
Ingredient |
Enter the names of all Single Ingredients, Compound Ingredients (or sub-recipes), and Components of Compounds The names of all ingredients (including Components of Compounds) must match valid ingredient names held in the Ingredient Glossary. Validation will be applied when using Calculate & Create Ingredient List, when validating the specification or when attempting to progress the Specification to the next status. Any invalid ingredient names must be corrected before proceeding. Ingredients may be entered by selecting from the Glossary: Click the ingredient icon and then either select an Ingredient Type or type part of the Ingredient Name into the Search field. Alternatively, Ingredient names may be freely typed into the Ingredient column. The predictive text facility presents a list of options for you to select from. If you type an Ingredient Alias (or alternative name for the Ingredient), the system automatically corrects this when the Ingredient List is calculated. The names of Compound Ingredients will not be found in the Glossary and may be freely typed. Validation errors will not be given for Compound Ingredient names. If the name of the Compound is to appear in the on-pack Ingredient List, be sure to enter it in the manner in which is should appear, including capitalization if applicable. Note: If you cannot locate an ingredient in the Glossary, even after considering possible alternative names and spellings, contact the System Administrator. |
|
Forced Addition |
When calculating the on-pack Ingredient List, the system automatically sums any Ingredients with identical names. Use the Forced Addition column to sum ingredients which do not have the same name, but need to be declared together in the Ingredient List, such as, Additive categories, Herbs and Spices, or re-constituted ingredients. You may select the grouping from the drop-down list or freely enter the name of the grouping into the field. Select or enter the same grouping next to each Ingredient which needs to be included in the grouping. If freely entering the name of the grouping, ensure that the exact wording is used for each ingredient to be summed; the system assists by presenting a list of previously entered items as you enter the name. Note: The name of the Grouping will appear in the on-pack Ingredient List (followed by the grouped ingredients in brackets), so it should be entered in the manner in which it is expected to appear, including capitalization if applicable. |
|
Quantity in Comp |
Enter the breakdown of all Compound Ingredients (or sub-recipes) into this column. The breakdowns of each Compound may be entered using any unit of measure, such as % breakdown, kilos per sub-recipe batch, and so on, as long as the same units are used within each individual Compound. One particular Compound may be expressed in different units to another. |
|
% in Comp |
The percentage breakdown of each Compound ingredient. This is automatically calculated by the system when selecting Calculate & Create Ingredient List. |
|
Batch Qty |
Enter the quantities of all Single Ingredients or top level Compound Ingredients into this column. Any unit of measure may be used, such as % breakdown, kilos per batch, or g per pack, but the same unit must be used throughout this column. |
|
Batch % |
The percentage breakdown of the Quantities in Batch. This is automatically calculated by the system when using Calculate & Create Ingredient List. |
|
Total % |
The percentage breakdown of all Declared ingredients, including adjustment for Ingredients which have been summed together (either by name or by Grouping) and for any water losses applied. This is automatically calculated by the system when using Calculate & Create Ingredient List. |
|
Dec |
Check the box to indicate all ingredients which must be declared in the Ingredient List. For Ingredients which are summed (either by name or by Grouping), only check the first occurrence of that Ingredient (the system prevents you from checking multiple occurrences of summed ingredients). For Compound Ingredients:
|
|
QUID |
Use this column to indicate any ingredients which are required to display a quantitative value in the Ingredient List. For any checked ingredients, the % value appears next to that Ingredient in the Ingredient List. |
|
Supp |
This column displays the number of Raw Material Suppliers which have been entered for each Ingredient. Click the icon to access the Raw Material Data Entry window. Add rows to the table for each of the number of Suppliers for this ingredient (separate entries must be added for each supplier) and enter the relevant details from each Supplier's Ingredient Specification. When OK is clicked, updates the number of Suppliers displayed in this column and the Raw Material tab with the details you have entered. Note: The Raw Material details may be entered directly in the table in the Raw Materials tab or using the Raw Material Data Entry window, described above, and accessed by clicking the icon in the Supp Column in the Recipe table. However, the number of Supplier rows may only be added or removed using the Raw Material Data Entry window. The last row (Supplier) for any Ingredient may not be removed. |
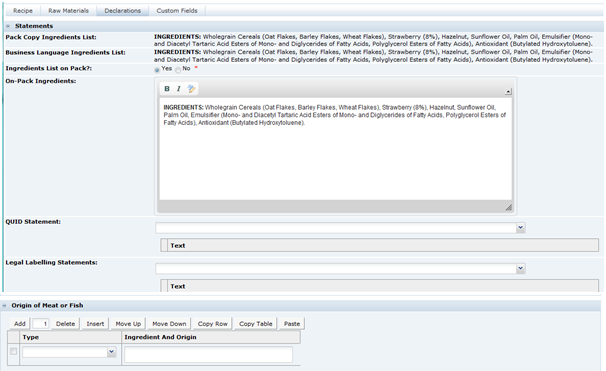
|
Pack Copy Ingredients List |
When Calculate & Create Ingredient List is clicked, the system generates an ingredient list based on the entered recipe. This ingredient list is shown as read-only in this field. It may be edited within the Declarations tab. |
|
Comments |
Click the Comments tab below the recipe to replace the Ingredients list by a comments free text field so any explanatory information can be added regarding the recipe. Click Ingredients to return to seeing the ingredient list. |
Single Ingredients, Compounds, and Components
The 1 and 2 columns in the Formulation table are used to indicate any compound ingredients and their components:
-
Use the 1 column to indicate Compound Ingredients: Use a simple numbering system, for example, the first compound in the formulation = 1, second compound = 2, and so on
-
List the Component Ingredients directly below the name of the Compound Ingredient and use the 2 column to indicate the number of the compounds to which they belong, such as, 1, 2, and so on. For example, see Figure 3-19.
-
Nested Compounds, that is, where a Compound or Sub Recipe contains Compound Ingredients itself, will have an entry in both the 1 and 2 columns, for example, Compound 2a, Component of Compound 2, and so on. For example, see Figure 3-19.
-
Leave the 1 and 2 columns blank for Single Ingredients, added directly to the final batch.
The system enables Suppliers to demonstrate any water lost from the recipe as part of the cooking process, with the effect that the water ingredient may move further down the ranking in the finished Ingredient List.
To achieve this, add suitable entries in the recipe table for Water, with a negative quantity to demonstrate the required water loss. The water-loss should be placed in the appropriate place in the recipe, for example, if a compound if is pre-cooked and water is lost in that compound, the negative water should be included as part of the compound. If it is lost in the final complete recipe through cooking of the whole product, it should appear as an additional ingredient outside of the compounds. Complex recipes may have multiple water addition and water loss entries, but each entry must use the same ingredient name Water to enable the system to sum them all correctly.
Calculate & Create Ingredient List Button
Once a recipe is completed, the Calculate & Create Ingredient List button is used to generate the Ingredient List. If any changes are made to the recipe, the button must be clicked again before the specification may be progressed to a different status or the Pack Copy is issued. This forces the ingredient list to be recalculated. The need to recalculate is indicated by the words on the button being changed from black to a red font.
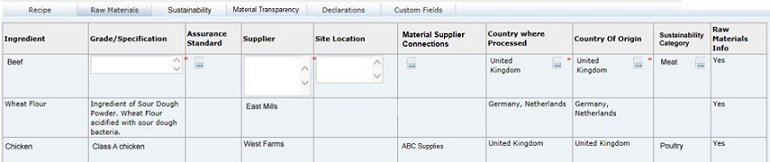
Raw Materials Tab
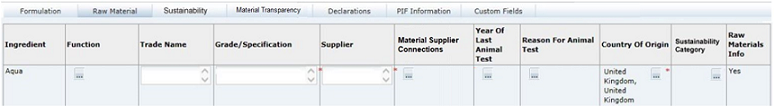
This tab provides a list of all Ingredients which have been entered into the Recipe table, with multiple rows for ingredients which have more than one Supplier. Additional details, such as Grade / Specification and Origin details, can be entered for each Ingredient and each Supplier.
The Raw Material details may be entered directly in the table in the Raw Materials tab or by using the Raw Material Data Entry window, accessed by clicking the icon in the Supp column in the Recipe table. However, Supplier rows may only be added or removed using the Raw Material Data Entry window.
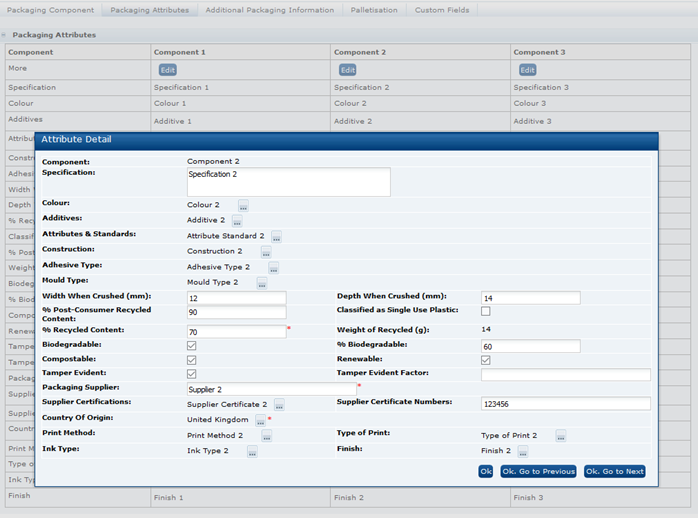
Click the icon in the Supp column of the Recipe table to access the Raw Material Data Entry window. Add rows to the table to represent the number of Suppliers for this ingredient (separate entries must be added for each supplier) and enter the relevant details from each Supplier's Ingredient Specification.
When OK is clicked, the Raw Material tab is updated with the details you entered.
Note:
The last row (Supplier) for any Ingredient may not be removed. The system defaults to requiring one row per Ingredient.Table 3-14 describes the fields.
Table 3-14 Food Specification Raw Materials Page
| Field | Description |
|---|---|
|
Ingredient |
Name of Ingredient entered in the Recipe table. Populated automatically by the system, with multiple rows for Ingredients which have more than one Supplier. |
|
Grade / Specification |
Free text field to enter specific details about the nature of the Ingredient, usually obtained from the Raw Material Specification. |
|
Assurance Standard |
Choose any options which apply to the raw material. |
|
Supplier |
The name of the raw material supplier. |
|
Site Location |
Location of the supplier's site from which the product is supplied. |
|
Country Where Processed |
Select the country or countries where the raw material undergoes further processing. For un-processed materials, this may simply be the place where they are packaged and shipped. Click the Country icon. Use the search option to locate the required country. Use the check boxes to select multiple countries, if necessary. |
|
Country of Origin |
Select the country or countries where the raw material is grown and harvested, or reared. For Compound ingredients, the Country of Origin may be the same as the Country where Processed. Click the Country icon. Use the search option to locate the required country. Use the check boxes to select multiple countries, if necessary. |
|
Sustainability Category |
Select a sustainability category to indicate if ingredients fall into particular categories of concern regarding sustainability. Typical examples are Meat, Fish, Poultry, and Palm Oil. The selection applies to all raw material rows for the ingredient. This column will only show if the Sustainability feature is enabled for Food specifications. |
|
Material Supplier Connections |
If the ingredient is a sub component, select the suppliers who use that ingredient from a list of the immediate parent ingredient's suppliers. This column provides the material transparency data used to form the structure of the supply chain, that is, the specific supplier relationships from one nested ingredient level to the next. The hierarchy is based on the compound and sub ingredient relationship references in the Recipe table. The picklist will only contain values for selection where the row is for a sub component ingredient (column 2 in the Recipe table is populated with a value that associates it to a parent compound), and the supplier of the parent compound has been populated accordingly; else there will be nothing to select. Note: This means that you should complete the supplier details of the top-level compounds first, and then work down through any nested levels, so that the Material Supplier Connections picklist is populated. The Set all Material Supplier Connections button can be used to automatically complete the Material Supplier Connections column (overwriting any that have been manually completed). This option applies to the common scenario of a one-to-one relationship where the sub component's parent ingredient has a single supplier assigned to it. The generated values may then be individually amended if necessary, or to set the more complex many-to-many relationships. If an ingredient or its supplier is removed from the Raw Materials table, any usage of the supplier as a connection in a sub component will be automatically adjusted, by removing the supplier reference from the associated ingredient's Material Supplier Connections column. The specification validation checks sub component ingredients for the presence of a Material Supplier Connections value based on the setting of the Material Transparency Mandatory Status system parameter, which defines at what status the value becomes mandatory (by default, Collaborative Draft status is assumed). |
|
Raw Materials Info |
Displays the Yes/No option which has been selected for the Raw Material Info Required field in the Raw Material Data Entry window. If set to No, the mandatory fields for that ingredient are hidden. |
Note:
The Material Supplier Connections value can also be selected in the Raw Material dialog data entry box, where the Ok. Go to Next and Ok. Go to Previous buttons provide a means of scrolling through the ingredients in the Recipe table, for completing the Material Transparency and other columns without having to return to the Recipe or Raw Materials tables.When paging to another row in edit mode, the present row's values are updated in the Raw Materials table, and the dialog box is refreshed to show the details of the selected ingredient row.
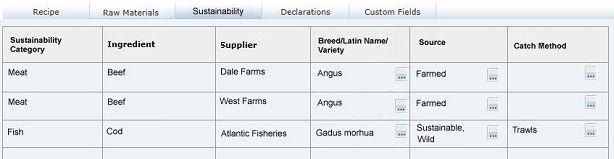
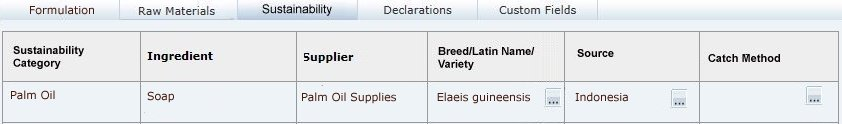
Sustainability Tab
This tab contains the Sustainability data, in a table which is automatically populated with a row for each ingredient in the Raw Materials table where a sustainability category has been assigned. The rows are presented in the order they appear in the Raw Materials table. If the ingredient has more than one supplier in the Raw Materials table, a row will be present for each supplier.
Note:
This tab only appears if the Sustainability feature is enabled for Food specifications.Table 3-15 describes the fields.
Table 3-15 Food Specification Sustainability Page
| Field | Description |
|---|---|
|
Sustainability Category |
The Sustainability Category entered in the Raw Materials table. Populated automatically by the system, with multiple rows for ingredients that have more than one Supplier. |
|
Ingredient |
Name of Ingredient entered in the Raw Materials table. Populated automatically by the system, with ingredients that have a sustainability category assigned, with multiple rows for ingredients that have more than one Supplier. |
|
Supplier |
The name of the Supplier entered in the Raw Materials table. |
|
Breed/Latin Name/Variety |
Select the Ingredient's breed, Latin name or variety, if relevant. The available selections are filtered to just show those that are associated to the ingredient's sustainability category. |
|
Source |
Select the Ingredient's source, if relevant. The available selections are filtered to just show those that are associated to the ingredient's sustainability category. |
|
Catch Method |
Select the Ingredient's catch method, if relevant. The available selections are filtered to just show those that are associated to the ingredient's sustainability category. |
Note:
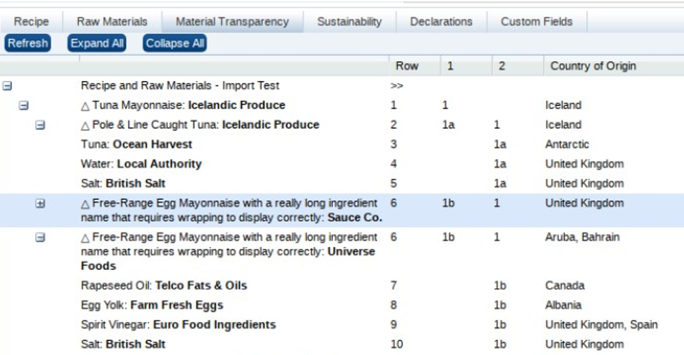
If an ingredient's sustainability category is changed in the Raw Materials table, the contents of the Breed/Latin Name/Variety, Source, and Catch Method columns will be cleared.Material Transparency Tab
This tab contains the Material Transparency tree view, a hierarchical representation of the raw materials supply chain.
Note:
This tab only appears if the Material Transparency feature is enabled for Food specifications.The rows of the list view are based on the ingredients in the Recipe and Raw Materials tables, grouped according to their ingredient, compound, and sub component nesting within the recipe.
Where there are multiple ingredient to supplier connections/relationships, and where the ingredient has more than one Material Supplier Connections supplier, the row is repeated, replicating the branches of the structure within the appropriate level of the tree.
The nested structure of the ingredients, compounds, and sub components is represented by indentation.
Where a row groups sub components, the next level of the tree can be expanded or collapsed using the + and - controls.
Buttons to expand or collapse all levels, and to refresh the view appear at the top of the page. By default, the rows are collapsed; multiple branches may be expanded at the same time. Options to expand or collapse all levels, and to refresh the view are also available by right-clicking the list view and selecting from a menu.
The uppermost root of the tree is the name of the specification (plus section name, if applicable), which remains expanded in the default position.
An icon identifies the type of item:
-
Triangle: Ingredient is a compound ingredient.
-
Diamond: Ingredient is not a compound (it is at the bottom of the structure), but has multiple suppliers within that branch of the tree.
-
No icon: Ingredient is neither a compound nor has multiple suppliers.
After its initial presentation, if changes are made to the Raw Materials table, the Refresh button or action must be used to see the changes in the tree view.
Double-clicking a row opens the Raw Materials dialog box in read mode, presenting the ingredient's raw materials and material traceability (and sustainability if present) as per clicking the Supp button in the Recipe table.
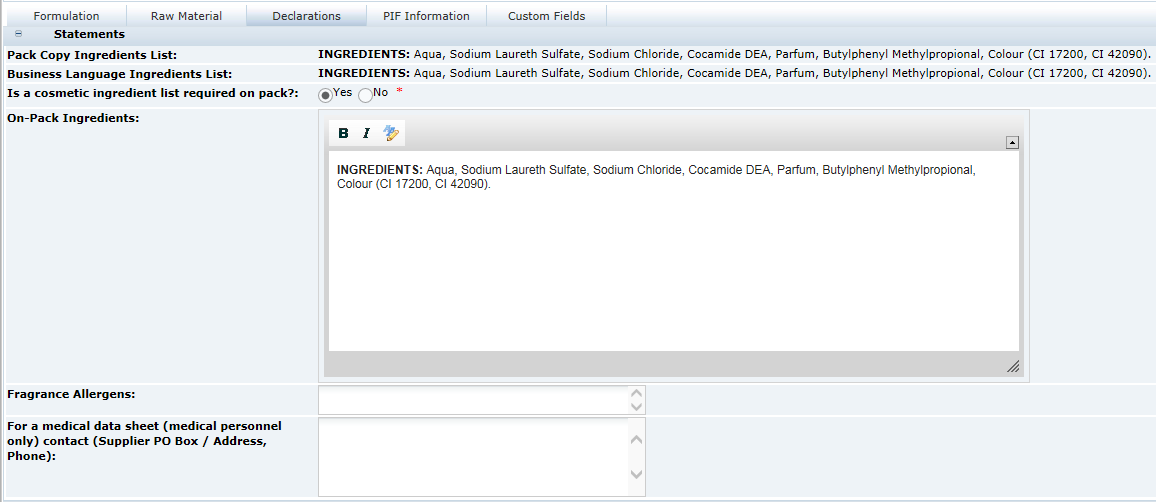
Declarations Tab
This tab contains all the fields that are related to the Ingredient list that are to be included in the Pack Copy, that is, the ingredient list, quantitative ingredient declaration statements, additional labelling statements required by legislation, and raw material origin statements.
Table 3-16 describes the fields.
Table 3-16 Food Specification Raw Materials Page
| Field | Description |
|---|---|
|
Pack Copy Ingredients List |
This is the ingredient list as it is generated by the system in the language of the Pack Copy. It is read only and cannot be changed. |
|
Business Language Ingredients List |
This is the ingredient list as it is generated by the system in the Business Language of the system, that is, the default language. It is read only and cannot be changed. |
|
Ingredients List on Pack? |
Default is Yes. If No is selected, the following field is hidden and no ingredient list is included in the Pack Copy. |
|
On Pack Ingredients List |
When the ingredient list is generated by the system, a copy is made in this field. It may then be edited manually to include additions to the ingredients list, such as, adding Organic to Carrots to make the ingredient Organic Carrots. This edited version of the ingredient list is the one that is used for the Pack Copy. It should be noted, that significant changes should not be made such as changing the order of ingredients, or adding or removing ingredients. The recipe should be amended accordingly if such changes are required. |
|
QUID Statement |
Certain Quantitative Ingredient Declaration type phrases that are required on pack may be selected here. Some phrases may require additional added text to be added in text boxes that are included in the phrase. Example: a cooked meat may have a statement to say that 100 g of the cooked meat is made with xx g of yyyy. Where xx is the amount and yyyy is the type of meat, such as Pork. These details are added by the user. |
|
Legal Labelling Statements |
Some products are required by legislation to have additional statements about their ingredients made on the pack, for example, a statement about fruit content on jam labels. Such statements are included here. |
|
Origin of Meat or Fish |
Statements about the origin of specific ingredients may be made here, using a combination of predefined phrases from the Type field and free text from the Ingredient and Origin field. Additional rows for each statement may be added to give the full original declaration that is required. Example: Using Tuna caught from the Indian Ocean. |
Note:
In release 17.0, the size of the Ingredients List, Business Language Ingredients List, and On Pack Ingredients List fields are reduced to 4,000 characters. For specifications that were created prior to release 17.0, the full 11,000 characters content of these fields can be viewed in the Change History log, and accessed using searches or reports.Custom Fields Tab
A Retailer may add some additional Custom fields that are required for the Recipe or Raw Material section. These Custom fields appear in this tab. If no Custom fields are set up, the tab is not seen.
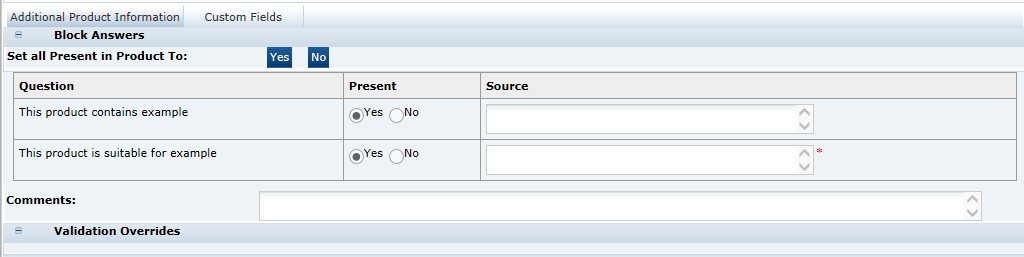
Allergy and Dietary Advice Section
This section of the specification is used to describe the presence or absence of allergens, other sensitive ingredients and the suitability of the product for certain dietary lifestyles. The Retailer may configure the system to ask for details about any allergen, other sensitive ingredients or dietary lifestyles that they may wish to gather information on and thereby impart to their customers.The section is used to define specific declarations required to be included in the Pack Copy.
This section has the following tabs:
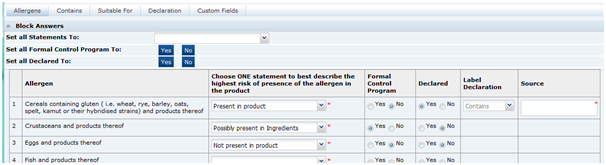
Allergens Tab
This tab contains a list of Allergens about which the Retailer wishes to gather information. The specification writer is required to provide information for all the allergens listed.
To assist in the completion of the tab, all statements may be answered with one block answer, an option to choose one statement for all the allergens, one answer to the Formal Control Program question, and one Declared answer. All the individual allergens may then be amended accordingly.
Table 3-17 describes the fields.
Table 3-17 Food Specification Allergens Page
| Field | Description |
|---|---|
|
Allergen |
The name or description of the allergen. |
|
Choose ONE statement to best describe the highest risk of presence of the allergen in the product |
For each allergen the most appropriate statement is selected from the following:
These statements are put into the considered order of risk to the consumer that is allergic to the allergen in question. |
|
Formal Control Program |
The question should be answered as to whether there is a formal control program in place to negate any risk identified above. |
|
Declared |
This defines if the system is to create an allergen statement within the Declarations tab that is to be included on the Pack Copy. |
|
Label Declaration |
A suitable statement beginning is selected if the Declaration is set to Yes. The options here depend on the previous answers provided. Often there may be only one option that can be selected. |
|
Source |
When the allergen is set to Present in Product, this field is used to explain what is the source of that allergen. In some cases, this may not be obvious from the recipe. |
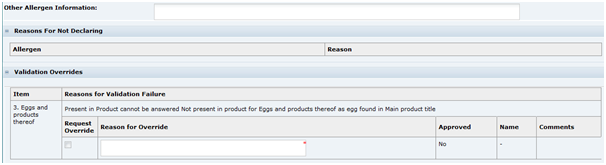
Beneath the list of allergens are fields for additional information.
Table 3-18 describes the fields.
Table 3-18 Food Specification Other Allergen Information Page
| Field | Description |
|---|---|
|
Other Allergen Information |
This provides the supplier with a space to provide any additional or explanatory information regarding allergens in the product. |
|
Reasons For Not Declaring |
There are options in the configuration to enforce that the user provides a reason for not declaring an Allergen that is said to be Present in the Product. In this case, if declared is set to No for any Allergen that is marked as Present in Product, this table lists those allergens and the Reason field is to be completed by the user. |
|
Validation Overrides |
When an allergen is marked as being Not Present in Product, and the Specification is moved to another status, a validation is carried out to check whether ingredients containing those allergens are present in the product's recipe or ingredient list or named within the Main Product Title or Legal Title in the Other Labelling information section. If they are found, the allergen cannot be set to Not Present in Product. However, in certain circumstances, this may still be the correct response. For example, a product may be called an Easter Egg, but it probably does not contain any egg or egg derivative. In such cases, a Validation Override is requested. The reason for this override is entered into the Reason for Override field and the specification may then be progressed to the next status. A Retailer user with the correct permission level is then required to approve the override by checking the Approved Checkbox and provide the reason, before the Pack Copy can be issued to the Artwork Designer. Note: The system has configuration options that the Retailer may set up to prevent certain common phrases from causing such validation failures. For example, the system could be set up to not fail the egg validation if it finds egg in the phrase Easter Egg. |
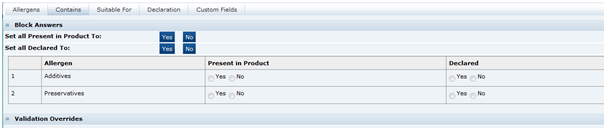
Contains Tab
This tab contains a list of sensitive ingredients that the retailer wishes to specifically gather information about their presence. For example, a retailer may wish to have information about whether certain preservatives are included in the product (not necessarily allergens). While this information may be gathered from the recipe, this tab allows simple reporting of which products contain particular ingredients. The tab is a simpler version of the Allergens tab.
Table 3-19 describes the fields.
Table 3-19 Food Specification Contains Page
| Field | Description |
|---|---|
|
Present in Product |
The answer to whether the product contains the particular ingredient. |
|
Declared |
Whether the ingredient is declared or not. Depending on the configuration of the system, a declared statement may be generated by the system in the Declarations tab to be included in the Pack Copy. |
|
Validation Overrides |
This functions similarly to the equivalent fields in the Allergens tab. |
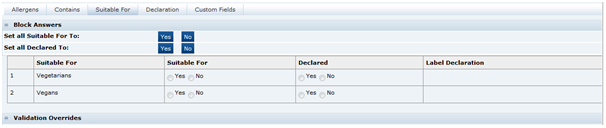
Suitable For Tab
This tab provides information as whether the product is suitable for certain dietary lifestyles listed, such as Vegetarianism, Kosher, and so on, or other lifestyles for which the Retailer wishes to gather information.
Table 3-20 describes the fields.
Table 3-20 Food Specification Suitable For Page
| Field | Description |
|---|---|
|
Suitable For |
The answer to whether the product is suitable for the dietary lifestyle described. |
|
Declared |
Whether the suitability is to be declared or not. A declaration statement may be generated in the Declarations tab to be included in the Pack Copy. |
|
Label Declaration |
The starting text is selected for the declaration statement to be created by the system in the Declarations tab. |
|
Validation Overrides |
This functions similarly to the equivalent fields in the Allergens tab. The system validates to check that certain ingredients are not included in the product that would render the product unsuitable for the dietary lifestyle. For example, Beef in a vegetarian product, though an override may be requested if Beef Tomatoes have been used. |
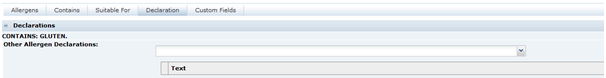
Declarations Tab
This tab has any declarations that have been generated by the system following completion of the other tabs in this section. All details in this tab are included in the Pack Copy. The statements generated by the system are read-only.
Table 3-21 describes the field.
Table 3-21 Food Specification Declarations Page
| Field | Description |
|---|---|
|
Other Allergen Declaration |
Suitable additional statements may be selected that are required on the label of the product. Some statements may require additional text to be added when the phrase is selected. Example: For allergens, please see ingredients in bold in the ingredients list. |
Custom Fields Tab
A Retailer may add some additional Custom fields that are required for the Allergy and Dietary Advice section. These Custom fields appear in this tab. If no Custom fields are set up, the tab is not shown.
Nutrition Section
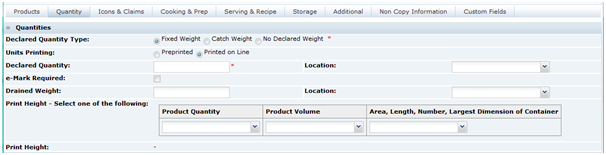
The Nutrition section is used to record the nutrition data for the product and, from this data, create a nutrition panel for the Pack Copy that complies with appropriate legislation regarding calculation of energy where relevant, rounding of nutrient levels, calculating percentages of reference intakes (or daily values), and presentation of the data.
The system may be configured for various legislations and product types. The fields shown for completion may vary depending on which legislation a product is being sold under (chosen when the specification was created), and the options selected within the section.
The figures shown below are for a European product sold under EU legislation, though references to US legislation are included.
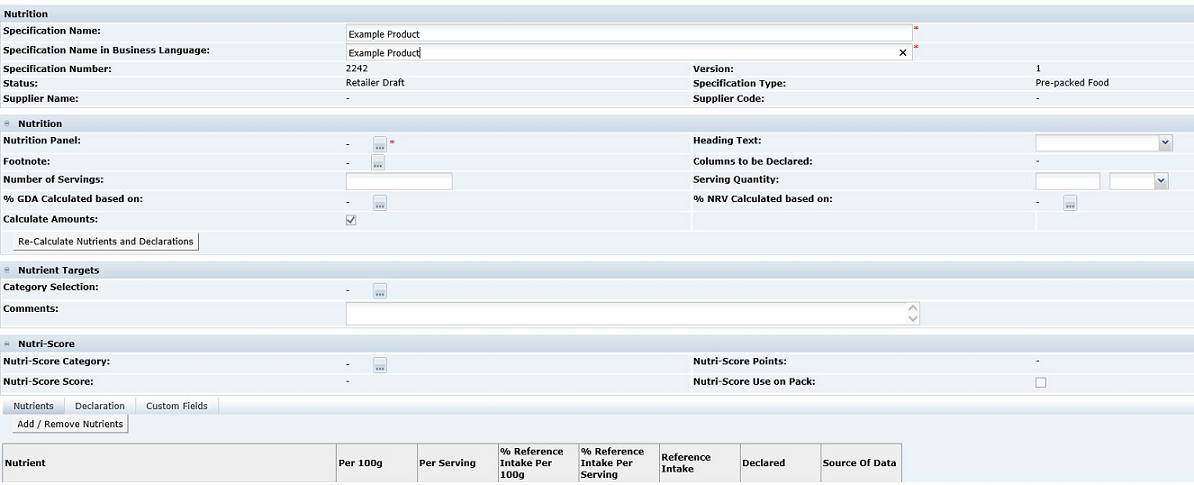
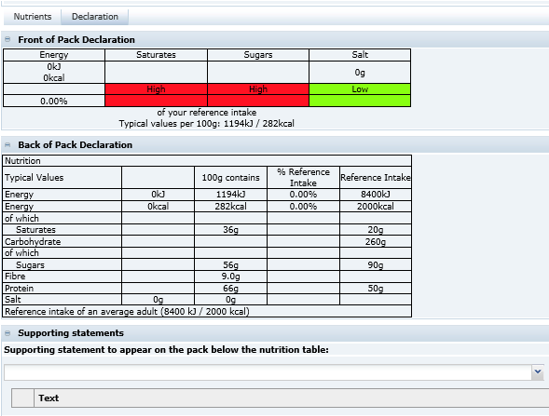
The section consists of a Nutrition Header. This is the first area beneath the main header, where the base detail for the nutrition declaration is completed.
When the Nutri-Score functionality is enabled, the next area contains the Nutri-Score details. The functionality is enabled by a system parameter. For details, see the Oracle Retail Brand Compliance Management Cloud Service Administration Guide.
Table 3-22 describes the fields in this area.
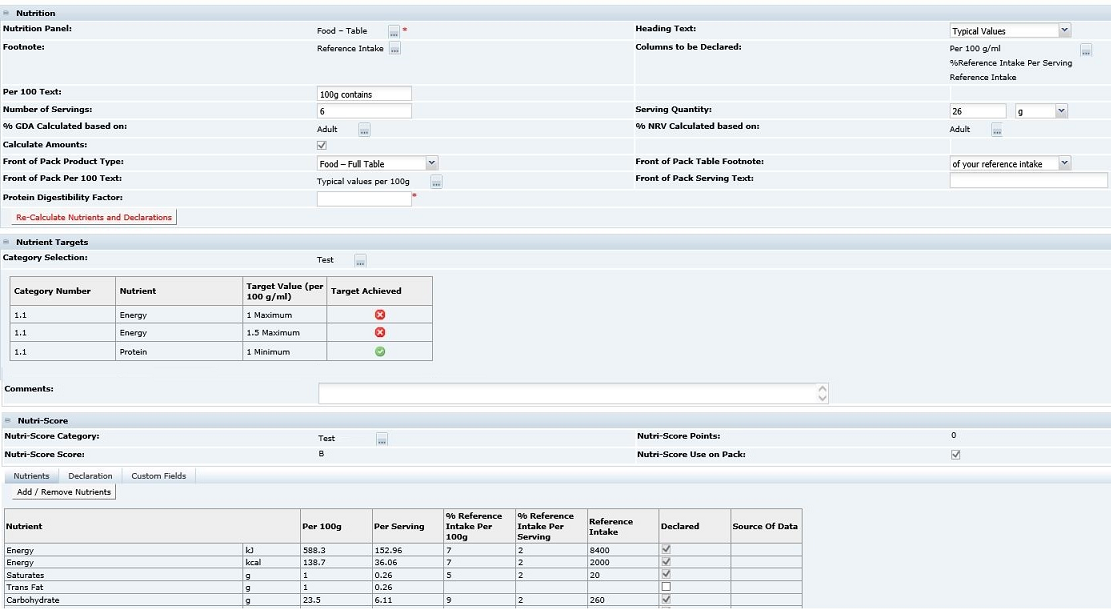
When the Re-Calculate Nutrients and Declaration button is selected and a Category has been assigned, the Points and Score fields are calculated. The configured nutrition values are used to calculate the total number of points based on the per 100g/ml nutrient values. The points are converted into the score. The Nutri-Score calculation is the same for all legislations.
Table 3-22 Nutri-Score Details
| Field | Description |
|---|---|
|
Nutri-Score Category |
This field contains the category for the specification's legislation. It includes the category number. This field is optional. |
|
Nutri-Score Points |
This read-only field shows the calculated points value. |
|
Nutri-Score Score |
This read-only fields shows the calculated score which is derived from the calculated points. |
|
Nutri-Score Use on Pack |
This checkbox determines if the Nutri-Score Score value is included in the specification's Pack Copy File. If checked, the Nutri-Score Score value is included. The initial default setting for this field is determined by a system parameter. For details, see the Oracle Retail Brand Compliance Management Cloud Service Administration Guide. |
This is followed by three tabs:
-
Nutrients
-
Declaration
-
Custom Fields
The starting point when completing the nutrition section, is to decide on the type of Nutrition Panel that is required on the pack. This is dependent on the type of product and, for some legislations, the target age group for the product.
Typical Nutrition Panels for a European product:
-
Food - Table: For most foods with nutrients, presented as a table.
-
Food - Linear: For most foods with nutrients, presented as text.
-
Supplements: For vitamin and/or mineral supplements with nutrients, presented as a table.
-
Pet-food: Primarily for cat and dog foods.
-
Bottled Water: For mineral contents of waters, presented as a table.
The following are some of the panels available for a US product, listed here to illustrate the possibilities:
-
Full food table
-
Dual food table
-
Infant Formula
-
Infant food
-
Children's food
-
Supplements
The available panels are seen when clicking on the selector button in the Nutrition Panel.
Figure 3-30 shows the fields after selecting the Food - Table in an EU specification (the most commonly used panel for EU foods).
After selecting the panel, all the fields that are required to be completed are shown. In many cases, a lot of the fields are pre-populated with default data that may then be changed if required.
Note:
The default data is configured by the Retailer and is the most commonly used data and therefore may not need to be changed. Those fields remaining blank are the ones that are very product specific.Table 3-23 describes function of all the fields with references to the EU and US type of nutrition panels, though other legislations are also supported.
Table 3-23 Food Specification Nutrition Page Example for EU Specification
| Field | Description |
|---|---|
|
What format is your laboratory information provided in? |
US only: This field is to let the Retailer know in what format the original data was gathered, that is, values per 100g or per 100ml. |
|
Nutrition Panel |
The panel type previously described. |
|
Heading text |
This is the heading text that is used in the nutrition panel, where it appears depends on the legislation. In most cases, the text is defaulted and there is only one option. In an EU panel, the text is Typical Values and in the US, Amount per serving. |
|
Footnote |
The type of predefined footnote is selected here. When the selector is clicked, a dialog box opens where multiple footnotes can be added using the table shown. |
|
Columns to be declared |
For some legislations, it is optional which data columns can be displayed. The selector here shows the available options. For other legislations, such as the US, the columns are fixed and no selector is available; therefore, no changes can be made. |
|
Per 100 text |
This free text field is for the text that is to head the per 100 column of the nutrition panel. This field is not used in US panels and so is hidden. |
|
Per serving text |
This free text field is for the text that is to head the per serving column of the nutrition panel. For a US food, this field is labelled Serving size and is used for the text that is used in the header of the panel to describe the serving size. |
|
Number of servings |
Used in both the EU and US style panels. In the EU, the number entered is presented in a footer. In the US, this data is presented in the header of the panel. |
|
Serving Quantity |
This field is split into two fields for the number and the units (g or ml). The number is used by the system to calculate the per serving data from the per 100 data (or vice versa). Note: This number is not used in the serving size description on the panel. |
|
% RI Calculated based on |
This field is used to define which values of nutrient reference intakes are to be used in the calculation of the %RI (%DV in US). For the EU, there is only one category currently, the Adult values specified in the Food Information Regulations. |
|
% NRV Calculated based on |
This field is used to define which values of reference intakes for vitamin and minerals are to be used in the calculation of the %NRV (%DV in US). For the EU, there is only one category currently, the Adult values specified in the Food Information Regulations. |
|
Calculate amounts |
This checkbox determines whether the system calculates the per serving values from the per 100 values using the Serving Quantity. If checked, the system calculates the values. If unchecked, the system does not calculate so that values can be manually entered (for example when the per serving is a cooked value and the per 100 is raw). |
|
Protein Digestibility Factor |
This field is only shown in a US specification if the % DV Calculated based on fields that is selected includes a Protein Claim. The protein digestibility factor is then entered into this field to adjust the %DV for the Protein in line with the legislative requirement. |
|
Source of Data |
The Supplier provides details of the sources of data for the different nutrients. |
|
The following fields are only shown for an EU panel where a UK Front of Pack Nutrition declaration is additionally required. |
|
|
Front of Pack Product Type |
Select whether the product is a food or drink. Options may also exist to have an Energy only declaration in this selector. |
|
Front of Pack Table Footnote |
Select from pre-defined footnotes for the front of the pack nutrition declaration. |
|
Front of Pack Per 100 Text |
The UK front of pack nutrition declaration is to include per 100 Energy values in the footnote. This field is used to select from pre-defined text to include with the values. |
|
Front of Pack Serving Text |
This free text field is used to describe the serving size that is required as a header for the front of pack nutrition declaration. |
Nutrients Tab
This tab is used to record the actual nutrient values. Table 3-24 describes the fields.
Table 3-24 Food Specification Nutrients Page
| Field | Description |
|---|---|
|
Add / Remove Nutrients |
When the Nutrition Panel was selected, the nutrients that are required to have values added, are listed in the Nutrients tab. All these nutrients must have values added. If additional nutrients are to be declared or values are to be provided without declaration, this button is clicked to show a dialog box to select the additional nutrients required. Note: The default nutrients, that were automatically listed when the nutrition panel was selected, cannot be removed and values must be added for these nutrients. Also, some panels may not have any default nutrients selected (such as Supplements), in which case all the nutrients required to be declared need to be selected here. |
|
Per 100 |
As the cursor is moved to each nutrient, a field opens to enable the user to enter the per 100 value. When a value is entered and the cursor is moved to another nutrient, the per serving value is calculated if the Calculate Amounts checkbox is checked (using the Serving Quantity value). Note: For the EU and certain other legislations, the Energy is calculated by the system and so the Energy values cannot be entered. For the US, they are not calculated and can be manually entered here. |
|
Per serving |
The per serving value is calculated, as previously described, if the Calculate Amounts checkbox is checked. If the checkbox is unchecked, the per serving value may be manually entered. Note: It is possible to enter the per serving value and allow the system to calculate the per 100 value, if the per serving data is the original source of data. |
|
Declared |
The declared column is a set of checkboxes used to define which nutrients are to be declared. Depending on the Nutrition Panel chosen, some nutrients will already have this box checked and the box cannot be unchecked (because the legislation requires that the nutrient is always declared), some will not be able to be checked (as the legislation does not permit the nutrient to be declared), and others will be able to be checked and therefore declared or left blank and undeclared. |
|
Re-Calculate Nutrients and Declarations |
This button within the Nutrition Header, is used to calculate certain values that are not calculated on nutrient data entry. It also updates the declaration that is to appear on the Pack Copy. When changes are made to any of the nutrient data or information in the Nutrition Header, the system forces the user to recalculate, using this button, before the status of the specification can be changed or the Pack Copy is issued. The need to recalculate is indicated by the words on the button being changed from black to a red font. |
|
% Reference Intake Per 100 |
After clicking Re-Calculate Nutrients and Declarations, using the per 100 value for each nutrient, the %RI (%DV in the US) per 100 is calculated based on the values selected in the % RI Calculated based on or'% NRV Calculated based on field as appropriate. |
|
% Reference Intake Per Serving |
After clicking Re-Calculate Nutrients and Declarations, using the per serving value for each nutrient, the %RI (%DV in the US) per serving is calculated based on the values selected in the % RI Calculated based on or % NRV Calculated based on field as appropriate. |
|
Reference Intake |
This column shows the reference intake (Daily Value in the US) for each nutrient, based on the values selected in the % RI Calculated based on or '% NRV Calculated based on field as appropriate. If blank, there is not a reference intake or Daily Value for that nutrient. |
|
The following additional fields are shown if a Dual panel is selected, that is, a nutrition panel that displays the as packaged and as prepared nutrient values. |
|
|
As Packaged Text |
This field is shown in the Nutrition Header. It is used to define the text to be used in the Packaged column heading of the Nutrition Facts panel. |
|
As Prepared/Served Text |
This field is shown in the Nutrition Header. It is used to define the text to be used in the Prepared column heading of the Nutrition Facts panel. |
|
Serving Quantity As Prepared |
This field is shown in the Nutrition Header.It is the equivalent of the Serving Quantity field, but is used to define the value to calculate the as prepared per serving values from the per 100 values. |
|
Per 100 As Prepared Per Serving As Prepared % Daily Value Per 100 As Prepared % Daily Value Per Serving As Prepared |
These four fields in the Nutrients tab are used for the as prepared data and are the equivalent of the Per 100, Per Serving, % Reference Intake Per 100, % and Reference Intake Per Serving previously listed, that are now used for the as packaged data where Reference Intake is replaced by Daily Values for the US. |
Declarations Tab
The Declarations tab displays the data that will be used on the Pack Copy document that is issued to the product's artwork designer. It shows a representation of the Front of Pack Nutrition declaration where there is one and the main Nutrition Declaration that is usually on the back of the pack. These are the declarations that have been generated from the fields as previously described, in the Nutrition Header and the Nutrients tab.
Note:
The order of the columns in panel outputs is controlled by the order set in the Available Columns table of the Nutrition Panel glossary record.There is one additional field in this tab that may be used if required. This is the Supporting statement to appear on the pack below the nutrition table field. This field is used to select pre-defined phrases that may be required to be displayed on the pack beneath the main nutrition panel. Some phrases may require additional text to be added in an included text box to complete the phrase.
Custom Fields
A Retailer may add some additional Custom fields that are required for the Nutrition section. These Custom fields appear in this tab. If no Custom fields are set up, the tab is not shown.
Lite Nutrition Panels
For certain legislations, the system has provision to have what are referred to as Lite panels, such as, Full Food Table - Lite (for the US). When one of these panels is selected, the system's automated calculation and rounding of data is bypassed so that a user can enter the data simply as it would appear on the pack, without any validation or checking to ensure that the values are valid or correct.
This type of panel is designed to be used where a Supplier already has an existing label as the source of the data. Using this panel obviates the need to back calculate in order to get the correct declaration. It is expected that a Retailer would only accept the use of this panel for existing products that were developed without the use of Oracle Retail Brand Compliance Management Cloud Service and that for new products, the normal calculated panel would be used to ensure that the correct rounding and calculation rules are applied by the system.
Finished Product Standards Section
The Finished Product Standards (FPS) section specifies the finished product standards for the product, including Product Attributes, Physical Product and Package Standards, and Chemical and Microbiological Standards. This section is similar to the Formulated Non Food specification's FPS section.
Product Attributes Tab
This tab contains three subtabs within it, for the product as sold, as used and for details of the benchmarks.
Table 3-25 describes the fields in the pages for the three subtabs.
Table 3-25 Food Specification Product Attributes Pages
| Field | Description |
|---|---|
|
Project Attributes: As Sold and As Used |
Enter all qualitative attributes for the predefined attributes (nf, Appearance, Aroma, Texture, and Flavour). Following are the typical classifications for Green, Amber/Yellow, and Red:
|
|
Additional Attributes: As Sold and As Used |
Use the table to enter any further attributes which are tested for this product and not mentioned in the main Product Attributes table. |
|
Benchmarks |
Pulls through the Benchmark details which are present on any linked Product Records for reference. Any amendments to this information must be made on the Product Records themselves (however the data may only be edited by retailer). |
Physical Standards Tab
This tab is used to capture the details of any physical product and package standards, such as color, viscosity, and so on.
Table 3-26 describes the fields.
Table 3-26 Food Specification Physical Standards Page
| Field | Description |
|---|---|
|
Physical Quantitative Standards |
If physical testing is carried out, provide the details. Enter sufficient rows to the table for the testing which is carried out. For each Test, the fields for Minimum, Target, Maximum, Units, Method, and Frequency must be completed if a test has been entered. If sets of standards have been preconfigured, one may be selected from the Physical Standards glossary. Selecting from the glossary will overwrite the contents of the table (following a warning prompt). If standards are preconfigured in the glossary, one of them may have been designated the default set, in which case the table will be automatically populated with that standard when creating a new specification. If the table is populated with predefined values from the glossary, they may then be edited or removed, or additional rows added. If the Test column is populated, a value must be entered in all other columns. |
|
Other Physical Standards |
Provide the details of any other physical standards which are applied to the product or package. This rich text field enables formatting to be applied. |
Chemical Standards Tab
This tab captures the details of any chemical standards, such as pH, moisture, % salt, % oil, and so on.
Table 3-27 describes the fields.
Table 3-27 Food Specification Chemical Standards Page
| Field | Description |
|---|---|
|
Chemical Quantitative Standards |
If chemical testing is carried out, provide the details. Enter sufficient rows to the table for the testing which is carried out. For each test, the fields for Minimum, Target, Maximum, Units, Method, and Frequency must be completed if a test has been entered. If sets of standards have been preconfigured, one may be selected from the Chemical Standards glossary. Selecting from the glossary will overwrite the contents of the table (following a warning prompt). If standards are preconfigured in the glossary, one of them may have been designated the default set, in which case the table will be automatically populated with that standard when creating a new specification. If the table is populated with predefined values from the glossary, they may then be edited or removed, or additional rows added. If the Parameter column is populated, a value must be entered in all other columns. |
|
Other Chemical Standards |
Provide the details of any other chemical standards which are applied to the product or package. This rich text field enables formatting to be applied. |
Microbiological Standards Tab
This tab captures the details of any microbiological standards.
Table 3-28 describes the fields.
Table 3-28 Food Specification Microbiological Standards Page
| Field | Description |
|---|---|
|
Microbiological Classification |
A Retailer may configure standard microbiological standards for certain product types, for example, all pre-packaged sandwiches of a particular type may have a standard microbiological specification. A classification may be selected here. This then results in an additional table of standards being displayed where the Test, Target, Maximum, and Units are predefined and read-only and the respective Method and Frequency is then completed by the Supplier. |
|
Tests |
If microbiological testing is carried out, provide the details. Enter sufficient rows to the table for the testing which is carried out. For each test, the fields for Target, Maximum, Units, Method, and Frequency must be completed. |
|
Other Microbiological Standards |
Provide the details of any other microbiological standards which are applied to the product or package. |
Storage Section
The Storage section specifies the storage requirements of the product, including work in progress and the finished product. The section is split into three field sets.
Table 3-29 describes the fields.
Table 3-29 Food Specification Storage Details Page
| Field | Description |
|---|---|
|
Pack Coding from Date of |
Select the process stage from which the pack coding is applied. |
|
Period |
Select the time period which is applied to the pack date coding, for example, hours, days, or months. |
|
Format |
Select the format in which the Date Coding will be applied to the package. |
|
Pack Coding |
Select the Display Until Code statement and then complete the Number of hours, days, weeks, or months which will be applied. Check the box to confirm whether this information will also be applied to the Outer Case. Repeat the above details for the Product Durability Code. Additional rows may be added to the table if different date coding parameters could be applied under different circumstances, for example, for weekend production runs. Use the Comments field to indicate the reason for each parameter. |
|
Minimum Life Into |
Select the delivery point at which minimum life must be applied and, in the adjacent field, enter the number of hours, days, weeks, or months which will be applied. |
|
Format of Lot Code on Product |
Free text field to enter details of the on-pack lot coding method, including an example code and how this is deciphered. |
Table 3-30 describes the fields.
Table 3-30 Food Specification Work In Progress Page
| Field | Description |
|---|---|
|
Work in Progress |
Add sufficient rows to the table to accommodate any storage requirements of the product at different work in progress stages. For each Process Stage, enter the minimum and maximum times on storage, and the minimum and maximum temperatures. Use the free text Comments field to enter any additional relevant details. |
Table 3-31 describes the fields.
Table 3-31 Food Specification Finished Product Storage Page
| Field | Description |
|---|---|
|
Finished Product Storage |
Add sufficient rows to the table to accommodate any storage requirements of the finished packaged product. For each Stage, enter the minimum and maximum temperature of storage, and the minimum and maximum humidity of the storage area. Use the free text Comments field to enter any additional relevant details. For the Retailer Distribution Chain, enter the distribution chain into which the product is delivered. |
Process Controls Section
This section specifies the process controls which are applied during manufacturing and packing of the product. There are three subtabs in this section.
Process Steps is the first subtab. Use this field to provide an outline of the Process Steps, Units Operations, and Key Control Points in the manufacture of the product. Table 3-32 describes this field.
Table 3-32 Food Specification Process Steps Page
| Field | Description |
|---|---|
|
Process Steps |
This is a large free text field, enabling some formatting to be applied to present the process steps clearly. To format any text, highlight the text you wish to apply formatting to and click the appropriate icon from the bar at the top of the field:
|
Table 3-33 describes the fields.
Table 3-33 Food Specification Critical Control Points Page
| Field | Description |
|---|---|
|
Critical Control Points |
Add sufficient rows to the table for each Critical Control Point. Complete the free text fields for Process Step, CCP No, Hazard, Control Measures, Critical Limits, Monitoring Procedures, and Corrective Actions. |
Table 3-34 describes the fields.
Table 3-34 Food Specification Critical Control Points Page
| Field | Description |
|---|---|
|
Critical Quality Points |
Add sufficient rows to the table for each Quality Control Point. Complete the free text fields for Process Step, Legal / Quality Issue, that is, the type of issue, Control Measures, Tolerance, Monitoring Procedures, and Corrective Actions. |
Packaging Section
The Packaging section specifies the Packaging format and all packaging components and materials used for the product, including the retail pack and any secondary and tertiary packaging.
Packaging Components Tab
This tab captures all packaging components relating to the product. In addition, details of any Recycling Icons which are to appear on the retail package can be stated.
Table 3-35 describes the fields.
Table 3-35 Food Specification Packaging Components Page
| Field | Description |
|---|---|
|
Packaging Description |
Free text field, in which to provide a summary description of the packaging format, describing the retail pack and any secondary and tertiary packaging. |
|
Packaging Components Table |
|
|
Component Description |
Select the packaging item from the pick-lis. Add sufficient rows to the table to enable all packaging items to be added for the retail pack and any secondary and tertiary packaging. |
|
Level |
Select one of the following:
|
|
Food Contact |
Means is the product suitable for food use. Select Yes or No. |
|
Specification |
Free text field to enter the pertinent details of the Packaging specification. |
|
Component Weight |
Numeric field. Enter the weight of the item (usually in grams). |
|
% Recycled |
Numeric field. Enter the percentage of recycled material. |
|
Print Method |
Free text field to enter the details of the method of printing. |
|
Weight of Recycled |
Numeric field. Enter the weight of the recycled content (usually in grams). |
|
Packaging Supplier |
Free text field to enter the name of the packaging supplier. |
|
Country of Origin |
Select the Countries of Origin of the component. |
|
Certification |
Free text field to enter the details of any certification numbers that may apply. |
|
Material |
Select the Material for the component. |
|
Recycling Icons |
|
|
Recycling Icon Text |
Add sufficient rows to the table for any Recycling Icons which could be included on the product package. Indicate any that might apply. Use the pick-list in the Recycling Icon column to select the name of the Icon. |
|
Supporting Text |
Use the free text field to add any Supporting Text which needs to appear in conjunction with the Icon on the package. |
|
Use on Pack Location |
Choose the appropriate option from the Use on Pack Location pick-list to indicate whether the Icon is to appear on the package and whereabouts. |
|
Component |
Only present in Multi Pack Product Specifications. When entering details of Recycling Icons, select the name of the Components which each Icon should be applied to, or Parent if the Icon is to appear on the outer package. This avoids the need to add multiple Packaging sections in a Multi-Pack Product specification in that the only differences are the requirements for Recycling Icons. You can accommodate all packaging items for the composite product in the Parent section. |
Palletisation Tab
Table 3-36 describes the fields.
Table 3-36 Food Specification Palletisation Page
| Field | Description |
|---|---|
|
Crate/Shipper/Dolly Type |
Select the applicable option. |
|
Units per Case/Crate/Shipper Units per Consumer Pack Crates/Cases per Pallet Layer Layers per Pallet Pallet Height Total Cases Per Pallet Maximum Pallet Stack |
Numeric fields. |
|
Method of Pallet Wrap and Stabilization |
Free text field to enter details regarding the method of pallet wrap and stabilization. |
|
Comments |
Free text field to enter any other packaging details. |
Other Labelling Copy Section
This section captures all on-pack labelling requirements for the product, which have not already been provided in the Recipe, Raw Materials, Allergy, Dietary Advice, and Nutrition sections. Any details entered in this section are passed through to the Pack Copy file.
This section has the following tabs:
Products Tab
Table 3-37 describes the fields.
Table 3-37 Food Specification Products Page
| Field | Description |
|---|---|
|
Main Product Title |
Enter the full name of the product to appear on the front of pack or principle display panel. |
|
Product Legal Name |
Enter the legal or regulated description of the product. |
|
Product Range |
Enter the range description if there is one. |
|
Marketing Sub Copy |
Enter any other description that adds to the marketing of the product. |
|
Brand, Sub Brand, Brand Type, and Other Brand Details |
These are copied from the Main Details section. |
Quantity Tab
This tab contains different fields depending on the choice of the Declared Quantity Type.
Table 3-38 describes the fields.
Table 3-38 Food Specification Quantity as Fixed Weight Page
| Field | Description |
|---|---|
|
Units Printing |
Select whether the quantity declaration will be pre-printed on the Artwork or will be printed on-line. |
|
Declared Quantity |
Free text field to enter the Net Quantity in the format exactly as it is to appear on the pack. From the Use on Pack Location drop-down list, select where on the pack the declaration is to appear. |
|
e-mark required |
For EU products, check if an e-mark is required after the declaration. Ignore for other legislations. |
|
Drained Weight |
Free text field to enter the Drained Weight in the format exactly as it is to appear on the pack. From the Use on Pack Location drop-down list, select where on the pack the declaration is to appear. |
|
Print Height - Select one of the following |
Select a suitable option from one of the drop down selectors. The Print Height for the quantity declaration is then calculated and displayed below. |
|
Print Height |
Calculated from the selection above. |
Table 3-39 describes the fields.
Table 3-39 Food Specification Quantity as Fixed Weight Page
| Field | Description |
|---|---|
|
Units Printing |
Select whether the Units of the quantity declaration are to be pre-printed on the artwork or whether they will be printed on-line with the quantity. |
|
Catch Weight Range and Increments Information |
Enter the range of weights to be provided. This is for information only and would not be printed on the pack. |
|
Increments |
Complete the Increments table for products which are packaged in pre-determined increments. Add sufficient rows for each pre-determined increment and enter the increment weight and unit for each. |
When No Declared Weight selected for the quantity, no fields are displayed for the option.
Icons & Claims Tab
Table 3-40 describes the fields.
Table 3-40 Food Specification Icons & Claims Page
| Field | Description |
|---|---|
|
Logo |
Select the applicable option for the primary logo on the package. |
|
Icons / Flashes, Nutrition Icons, Taste Icons, and Other Icons |
Add sufficient rows to the table for any icons which could be included on the product package. Indicate all icons which might apply:
|
|
Nutrition Claims, Claims / Statements, Safety and Warning Phrases |
Select any claims which could appear on the product package. Indicate all Claims, Statements, or Phrases which might apply:
|
|
Environmental Phrases |
Select any Environmental Phrases that may be relevant to the product that are required to be included on pack. Choose the appropriate option from the Use on Pack Location drop-down list to indicate where the icon is to appear on the package. |
|
Serving Suggestion Required |
Select Yes or No to indicate whether the wording Serving Suggestion is to appear next to the picture of the product on the label. |
|
Air Freighted Product |
Answer whether the product is to ever be airfreighted. If Yes is selected, additional questions are to be answered. |
Cooking & Prep Tab
Table 3-41 describes the fields.
Table 3-41 Food Specification Cooking & Preparation Page
| Field | Description |
|---|---|
|
For all appropriate cooking methods |
Complete any specific time and temperature fields. Select the instructions from the drop down list. Repeat this action a number of times to build up the full instructions, in the order in which they should appear. The selected phrases will drop into the free text fields where they can be edited if necessary. |
Table 3-42 describes the fields.
Table 3-42 Food Specification Cooking Icons Page
| Field | Description |
|---|---|
|
Cooking Icons |
|
|
Cooking Warnings |
Select any warnings which are to appear on the product package. Indicate all Cooking Warnings which might apply:
|
|
Preparation Guidelines |
Free text field. If a product does not require cooking, provide any other preparation instructions exactly as they should appear on the pack. |
Serving & Recipe Tab
This tab captures any on-pack serving guidelines and recipe suggestions. The text may be formatted as it may be on the pack. Complete only the fields which are required on the package for this product.
Table 3-43 describes the fields.
Table 3-43 Food Specification Serving and Recipe Information Page
| Field | Description |
|---|---|
|
Serving Suggestions |
Free text; complete any Serving Suggestions exactly as it should appear on pack. |
|
Serves |
Enter the number of servings per pack, if this statement is required on the package. |
|
Recipe Suggestions |
Free text field. Complete any Recipe Suggestions exactly as it should appear on the pack. |
Storage Tab
This tab captures any on-pack Storage information. Complete only the fields which are required on the package for this product.
Table 3-44 describes the fields.
Table 3-44 Food Specification Storage Page
| Field | Description |
|---|---|
|
Storage Phrases, Home Freezing Guidelines, and Defrosting Guidelines |
Select any phrases which could appear on the product package. Indicate all Phrases or Guidelines which might apply:
|
|
Are standard frozen product storage guidelines required? |
Select Yes or No. |
|
Storage Icons |
|
|
Durability Coding |
Note: The date code statements selected here in Other Labelling Copy > Storage should correspond with the statements identified in the Spec's Storage section. |
|
Location of Durability Code |
Select whether the actual on-line-applied Durability Code will appear Alongside Statement or Elsewhere on Packaging. If Elsewhere on packaging is selected, enter the text which will appear adjacent to the statement, such as See Lid. |
Additional Tab
This tab captures any remaining information that should appear on the package. Complete only the fields which are required on the package for this product.
Table 3-45 describes the fields.
Table 3-45 Food Specification Additional Page
| Field | Description |
|---|---|
|
EAN / Barcode |
Enter the EAN or UPC barcode. If more than one code is to be used for this product, for example for promotions, additional rows can be added to the table. |
|
Shipping Case Code |
Enter any Shipping Case Codes, if applicable. |
|
Certificates |
Before working on Specifications, check the Site record for any Sites which are associated with this Specification and ensure that the References table has been completed. Check the Use on Label box for any of the items where the Certificate details would appear on product packaging. For information on the viewing the Site record, see the Oracle Retail Brand Compliance Management Cloud Service Supplier User Guide. Within the Specification, any of the References which have the Use on Label box checked in the Site record will automatically appear in the Certificates table. You must choose the appropriate option from the Use on Pack drop-down list to indicate what is to appear on this product package. When the specification's Pack Copy file is generated, the Certificates table will be populated accordingly: Use Both: Certificate Type, Certificate Number and Icon name Use Icon: Certificate Type and Icon name Use Number: Certificate Type and Certificate Number Do Not Use: None of the Certificates fields are populated |
|
Country of Origin Statements |
From the Statement drop-down lists, choose the applicable on-pack statement. Two Statement options are available, to accommodate products which require them. From the Country drop-down lists, choose the applicable country. |
|
Distribution Text |
Select the applicable option. |
|
Supplier Code on Pack and Site Code on Pack |
Select if either code is to be included in the pack. |
|
Copyright Year |
Free text field to enter the Copyright year of the label, if applicable. |
|
Price Box |
Defaults to Price Box Not Required. Change to an appropriate option if a Price Box is required on the package. |
|
Standard Price and Launch Promo Price |
Free text field to enter the price details, if applicable. |
|
Any other Information Front of Pack |
Free text field to enter any other information which might be needed or required on the front of the package. |
|
Any Other Information Back of Pack |
Free text to enter any other information which might be needed or required on the back or sides of the package. |
Non Copy Information Tab
Table 3-46 describes the fields.
Table 3-46 Food Specification Non Copy Information Page
| Field | Description |
|---|---|
|
Pack Copy to be Forwarded To |
This provides the email address to whom the Pack Copy files will be sent for this Specification. Select the required name from the list. |
|
Design Comments |
Free text field to enter any instructions or comments to brief or assist the artwork design. |
|
Reason for Issue |
Free text field to enter details of the reason for issuing the Pack Copy. Update each time the Pack Copy is issued. Provides details of the changes from previous versions to assist the artwork designer. |
|
Details of other documents to be sent separately |
Free text field to enter details of any other printed materials which form part of the product package. May be sent to the artwork designer separately to the Pack Copy files, such as style guides, sample design package, information leaflets, and so on. |
|
CAD Reference |
Reference to any CAD design. |
|
Cutter Guide Attachments |
This is a special area to attach any additional documents which need to be sent to Design at the same time as the Pack Copy files. |
Answer the various questions in the Other Details subsection regarding the artwork printing process where relevant. Add sufficient rows to the Printers table to provide details for each of the printers that are involved in printing the packaging for this product. Multiple rows may also be added per printer, if they handle more than one printed component, whereby each require different information to be given.
This part of the Non Copy Information is automatically populated by the system when the Pack Copy is issued.
Counter Ticket Section
For the Food Specifications that are of the type Counter Food, the Other Labelling Copy section is replaced by the Counter Ticket section.
This section is used to define the legal information that is printed on the small tickets that are displayed on the product on fresh counters to describe the product. It may also be used by some retailers to define additional information that is to be printed on the customer price label that is placed on the wrapped portion of product served to the customer, such as, allergen information.
As with the Pre-Packed Food, the Pack Copy document will contain any declarations defined in the Recipe & Raw Materials, Allergen & Dietary Advice, and Nutrition section plus the fields in the Counter ticket section.
The section is divided into three tabs or pages:
Product Details Tab
This section has the basic information about the product that may not actually be used on the Counter Ticket.
Table 3-47 describes the fields.
Table 3-47 Food Specification Counter Ticket Product Details Page
| Field | Description |
|---|---|
|
Counter Ticket to be forwarded to |
Provides the email address to whom the Pack Copy files will be sent for this Specification. Select the required name from the list. |
|
Reason for Issue |
Free text field to enter details of the reason for issuing the Pack Copy. Update each time the Pack Copy is issued. Provides details of the changes from previous versions to assist the artwork designer. |
|
Counter Type |
The drop-down list shows the different type of counters that the Retailer has. Select the counter that the product is to be sold through, such as, Deli counter or Pizzeria. |
|
Region |
If the product is to be sold only in certain regions, select the option that applies. |
|
Legal Price Marking Format |
How the product is to be priced, such as, per 100g, per kg, per item, and so on. |
|
Typical Weight of item (for price per item) |
If the product is to be priced per item, provide a typical weight. This may be included on the Counter Ticket. |
|
Product Technologist, Date Issued, Issued By, and Copy Brief Issue |
These fields are automatically populated by the system when the Pack Copy, sometimes also referred to as Copy Brief, is issued by the Retailer. |
Front of Ticket Tab
This tab is used to define the information that is to be included on the front of the Counter Ticket, that is, the side that is facing the customer.
Table 3-48 describes the fields.
Table 3-48 Food Specification Counter Ticket Front of Ticket Page
| Field | Description |
|---|---|
|
Sub Brand and Other Brand Details |
These fields are copied from the Main Details section if populated. |
|
Product Name |
The name of the product. |
|
Product Description & Legal Name |
Any additional legal description that is required in addition to the Product Name above. |
|
Website Product Description |
Add a description of the product to be included on the Retailers home shopping website if relevant. |
|
Additional Technical Copy for the Counter Ticket |
This table is used to provide details of any prescribed information that is to be included on the front of the Counter Ticket.
The selected information will be listed in the table of the section. The order can then be altered by using the Move Up and Move Down buttons. Note: Some information may require addition text to be added after the selection of the phrase. This is added into the text box in the table after the selection of all the information. |
Back of Ticket Tab
This tab is used to define information that is on the back of the ticket, typically instructions to the counter staff.
Table 3-49 describes the fields.
Table 3-49 Food Specification Counter Ticket Back of Ticket
| Field | Description |
|---|---|
|
PLU |
Enter the Price Look Up code. If there are to be multiple tickets produced with different PLUs, then add a row for each different code. |
|
Additional Information for Counter Staff |
This table functions in the same way as the Additional Technical Copy for the Counter Ticket table on the Front of Ticket tab. An example of the type of information that may be included here is the required Display until instruction. |
Advanced Packaging Section
The Advanced Packaging section provides an alternative to the Packaging Section allowing for the collection of more packaging data than the standard Packaging Section. Included are reporting capabilities and recycling information handling.
Packaging Components Tab
This tab captures key packaging components relating to the product with a link to add more packaging component details in a dialog window. In addition, optional Recycling Advice information can be automatically populated when configured to display and any Other Recycling Icons which is to appear on the retail package can also be stated here.
Table 3-50 Food Specification Advanced Packaging Components Page
| Field | Description |
|---|---|
|
Description |
Free text field, in which to provide a summary description of the packaging format, describing the retail pack. This field is mandatory. |
|
Packaging Components Table |
|
|
Component |
Select the packaging item from the pick-list. Add sufficient rows to the table to enable all packaging items to be added for the retail pack and any secondary and tertiary packaging. Picklist: from glossary - Component |
|
Level |
Select the level from the pick-list, for example, Primary, Secondary, Tertiary. The Level glossaries include a checkbox for Apply Recycling Advice Icon. Picklist: from glossary - Levels |
|
Material |
Select the packaging component material from the pick-list. Multiple materials can be selected to accommodate laminates. The Material glossaries incorporate a single-select picker for Base Material, a checkbox for Apply Recycling Advice Icon, and a multi-select picker for Recycling Advice Icon. Picklist: from glossary - Material |
|
Base Material |
This field is automatically populated from the Base Material selected in the corresponding Material glossary, for example, Plastic, Paper, Glass. Picklist: from glossary - Base Material |
|
Food Contact |
Means the product is suitable for food use. Select Yes or No. |
|
Component Weight (g) |
Mandatory numeric field. Enter the weight of the item (in grams). |
|
Rigid Component Weight (g) |
Optional numeric field. Enter the rigid weight of the item (in grams). |
|
Length (mm) |
Optional numeric field. Enter the length of the item. |
|
Width (mm) |
Optional numeric field. Enter the width of the item. |
|
Height (mm) |
Optional numeric field. Enter the height of the item. |
|
Flexible Component Thickness |
Optional numeric field. Enter a value for the item. |
|
Recycling Advice Table |
|
|
Component |
Read only - Auto-populated from Packaging Components table. |
|
Base Material |
Read only - Auto-populated from Packaging Components table. |
|
Recycling Advice Icon |
Select from the required Recycling Advice icons available. These are from the corresponding Material, for example, Widely Recycled, Check Locally, Not Yet Recycled. Picklist: from Material Glossary - Recycling Advice Icons |
|
Supporting Text |
Select additional recycling information text to be displayed on the packaging, for example, Cap on, Rinse, Flatten Bottle, Replace Cap, Remove Sleeve. Picklist: from glossary - Recycling Advice Supporting Text |
|
Use on Pack |
Choose the appropriate option from the Use on Pack pick-list to indicate whether the Icon is to appear on the package and whereabouts. |
|
Use Component |
Yes/No - Determines whether the Component name pulls to Pack Copy. |
|
Use Base Material |
Yes/No - Determines whether the Base Material name pulls to the Pack Copy. |
|
Multi-pack Component |
Only present in Multi Pack Specifications. Select the name of the Components which each Icon should be applied to, or Parent if the Icon is to appear on the outer package. |
|
Other Recycling Icons Table |
|
|
Text |
Add sufficient rows to the table for any Recycling Icons which could be included on the product package. Indicate any that might apply. Use the pick-list in the Recycling Icon column to select the name of the Icon. |
|
Supporting Text |
Use the free text field to add any Supporting Text which needs to appear in conjunction with the Icon on the package. |
|
Use on Pack |
Choose the appropriate option from the Use on Pack pick-list to indicate whether the Icon is to appear on the package and whereabouts. |
|
Component |
Only present in Multi Pack Product Specifications. When entering the details of the Recycling Icons, select the name of the Components which each Icon should be applied to, or Parent if the Icon is to appear on the outer package. This avoids the need to add multiple Packaging sections in a Multi-Pack Product specification in that the only differences are the requirements for Recycling Icons. You can accommodate all packaging items for the composite product in the Parent section. |
Packaging Attributes Tab
This tab allows for the capture of additional packaging component attributes relating to the product. The number of columns the table displays may be limited by a system configuration parameter.
Table 3-51 Food Specification Advanced Packaging Attributes Page
| Field | Description |
|---|---|
|
Component |
Read only automatically populated from the table on the Packaging Components tab. |
|
Specification |
General descriptive information, for example, gauge, quality, and type. |
|
Classified as Single-Use Plastic |
Check if component is a single-use plastic. |
|
Renewable |
Check if component is renewable. |
|
Compostable |
Check if component is compostable. |
|
Biodegradable |
Check if component is biodegradable. |
|
% Biodegradable |
Numeric field. Enter percentage value for how much of the component is biodegradable. Zero can be entered, to indicate the component is not biodegradable. |
|
Tamper Evident |
Check if component is tamper evident. |
|
Tamper Evident Factor |
Description of tamper evident qualities. |
|
Width When Crushed (mm) |
Optional numeric field. Enter the width of the item when crushed. |
|
Depth When Crushed (mm) |
Optional numeric field. Enter the depth of the item when crushed. |
|
% Recycled Content |
Numeric field. Enter percentage value for how much of the component is recyclable. Zero can be entered, to indicate the component is no recycled content. |
|
% Post-Consumer Recycled Content |
Numeric field. Enter percentage value for how much of the post-consumer component is recyclable. Zero can be entered, to indicate there is no recycled content. |
|
Weight of Recycled (g) |
This is a read-only field that is automatically calculated from the Component Weight and % Recycled. |
|
Additives |
Select the packaging component additives from the pick-list. Multiple options can be selected, for example, Lubricants, Antistatic Agents, Fungicides, Deodorants. Picklist: from glossary - Additives |
|
Attributes & Standards |
Select the packaging component attributes and standards from the pick-list. Multiple options can be selected, for example, Combined Disposal, Rigid, Recyclable, Compostable, FSC Certified, Biodegradable, PVC Free. Picklist: from glossary - Attribute Standard |
|
Colour |
Select the packaging component colour from the pick-list, for example, Clear, Green, Blue. Picklist: from glossary - Colour |
|
Construction |
Select the packaging component construction from the pick-list, for example, Laminate, Composite, Coextruded. Picklist: from glossary - Construction |
|
Adhesive Type |
Select the packaging component adhesive type from the pick-list, for example, Hot Melt, Solvent, Heat. Picklist: from glossary - Adhesive Type |
|
Mould Type |
Select the packaging component mould type from the pick-list, for example, General trade, special. Picklist: from glossary - Mould Type |
|
Print Method |
Select the packaging component print method from the pick-list, for example, Lithographic, Flexographic, Rotogravure. Picklist: from glossary - Print Method |
|
Type of Print |
Select the packaging component type of print from the pick-list, for example, Surface, Reverse. Picklist: from glossary - Type of Print |
|
Ink Type |
Select the packaging component ink type from the pick-list, for example, Cold Set, Water Based, Solvent Based. Picklist: from glossary - Ink Type |
|
Finish |
Select the packaging component finish from the pick-list. Multiple options can be selected, for example, Varnish, Foil.Picklist: from glossary - Finish |
|
Supplier Certificates |
Select the packaging component supplier certificates from the pick-list. Multiple options can be selected, for example, BRC, SEDEX. Picklist: from glossary - Supplier Certificates |
|
Supplier Certificate Numbers |
Details of any certificate numbers. |
|
Packaging Supplier |
Free text field to enter the packaging supplier. This is a mandatory field. |
|
Country of Origin |
Select the packaging component country of origin from the pick-list. Multiple options can be selected. Picklist: from glossary - Countries (existing glossary) |
Additional Packaging Information Tab
This section allows packaging-specific questions to be answered.
Table 3-52 Food Specification Advanced Packaging Additional Packaging Information
| Field | Description |
|---|---|
|
Question |
A read-only field automatically populated from a spec glossary (Additional Information Questions). |
|
Response |
Select a Yes or No response to the question. |
|
Component |
Select the packaging component the question is relevant to from the pick-list. The components will be those in the current specification. Multiple options can be selected. |
|
Comments |
Free text field to note a comment regarding the question. |
Palletisation Tab
This section is defined per the Food Specification. For more information, see the "Palletisation Tab" description for the Food Specification section.
Custom Fields Tab
This tab is defined per the Food Specification. For more information, see the "Custom Fields Section" description for the Food Specification section.
Project Links Section
From any part of the specification the specification may be linked to a an activity in Project, see the Oracle Retail Brand Compliance Management Cloud Service Project User Guide.
This section shows what links have been made. An action menu is available that shows the specific actions that may be taken regarding that link.
Custom Fields Section
A Retailer may set up a number of additional custom fields in this dedicated Custom section in order to gather additional information that the remainder of the specification does not adequately cover. This is additional to any custom fields that have been set up in other specific sections. If no such custom fields have been set up, this section of the specification is not shown.
Note:
The Retailer may set up different custom fields for each type of specification.Attachments Section
This section is used to attach any documents that may be required by the Retailer and that are relevant to this product specification. Attachments may be added within any section of the specification, they will all be displayed in this section.
The documents are attached by selecting New Attachment from the Actions menu. Browse the computer drives to find the document. The file name will be recorded, along with the size of the file, and the user's details. A suitable description is to be added to adequately describe the contents of the attachment.
Note:
An attachment may be a URL. If this option is selected, the name of the URL is entered along with a description. The attachment will then show as a hyperlink to allow the URL to be opened directly from the specification.Change History Section
This shows the change history for the Specification, including the dates of any status changes.
Formulated Non Food Specification
The Formulated Non Food (FNF) specification contains the following sections:
The workflow of the FNF specification and general specification management framework functionality, such as the relationship to Product Records and Pack Copy files, and the management of multi specifications is as described for the Food specification.
The core default Specification Type is Formulated Non Food. The Specification Name is mandatory for a new specification to be saved.
Main Details Section
The FNF specification's Header and Main Details section are as per the Food specification. For more information, see the "Main Details Section" description for the Food Specification section.
Formulation and Raw Materials Section
The Formulation and Raw Materials section enables Suppliers to provide details of the product formulation, with a breakdown of all ingredients, sub-recipes, and compound ingredients. This section is mandatory. The system automatically calculates the on-pack Ingredient Declaration based on the specification's selected legislation. This section is based on the Food specification's Recipe and Raw Materials section.
Formulations Tab
This tab provides details of the Formulation, all ingredients, compound ingredients (or sub-recipes), and component ingredients are listed, with quantities and whether they are to be declared.
When working in a new Specification, use Add, Insert, and Delete to add sufficient rows to the table for all of the ingredients in the formulation.
Table 3-53 describes the columns.
Table 3-53 FNF Formulation Page
| Column | Description |
|---|---|
|
1 |
This column is used to indicate primary Compound Ingredients. If this Ingredient is not a Compound Ingredient, leave the column blank. For more information, see "Single Ingredients, Compounds, and Components.". Alternatively, you can enter a number sign (#) into this column in order to insert a Comment row or blank row into the formulation that is not to appear on the label. This helps make reviewing a lengthy formulation easier. |
|
2 |
his column is used to indicate the Components of Compound Ingredients. If the Ingredient is not a Component of a Compound, leave the column blank. For more information, see "Single Ingredients, Compounds, and Components.". |
|
Ingredient |
Enter the names of all Single Ingredients, Compound Ingredients (or sub-recipes, pre-blends), and Components of Compounds. The names of all ingredients (including Components of Compounds) must match valid ingredient names held in the Ingredient Glossary. Validation will be applied when using the Calculate & Create Ingredient List button, when validating the specification or when attempting to progress the Specification to the next status. Any invalid ingredient names must be corrected before proceeding. Ingredients may be entered by selecting from the Glossary. Click the ingredient icon and either select an Ingredient Type or type part of the Ingredient Name into the Search field. Alternatively, Ingredient names may be freely entered into the Ingredient column. The predictive text facility will present a list of options for you to select from. If you type an Ingredient Alias (or alternative name for the Ingredient), it is automatically corrected when the Ingredient List is calculated. The names of Compound Ingredients are not found in the Glossary and may be freely entered. Validation errors will not be given for Compound Ingredient names. If the name of the Compound is to appear in the on-pack Ingredient List, be sure to enter it in the manner in which it should appear, including capitalization, as applicable. Note: If you cannot locate an ingredient in the Glossary, even after considering possible alternative names and spellings, contact your representative at the retailer. |
|
Forced Addition |
When calculating the on-pack Ingredient List, it automatically sums any Ingredients with identical names. Use this grouping column to sum ingredients which do not have the same name, but need to be declared together in the Ingredient List. You may select the grouping from the pick-list or freely enter the name of the grouping into the field. Select or enter the same grouping next to each Ingredient which needs to be included in the grouping. If freely entering the name of the grouping, ensure that the exact wording is used for each ingredient to be summed. A list of previously-entered items is presented as you type. Note: The name of the Forced Addition will appear in the on-pack Ingredient List, so it should be entered in the manner in which it is expected to appear, including capitalization if applicable. |
|
Qty in premix |
Enter the breakdown of all Compound Ingredients (or sub-recipes) into this column. The breakdown of each Compound may be entered using any unit of measure, such as % breakdown, kilos per sub-recipe batch, and so on, as long as the same units are used within each individual Compound. One particular Compound may be expressed in different units to another. |
|
% in Premix |
The percentage breakdown of each Compound ingredient. This is automatically calculated when Calculate & Create Ingredient List is clicked. |
|
Batch Qty |
Enter the quantities of all Single Ingredients or top level Compound Ingredients into this column. Any unit of measure may be used, such as % breakdown, kilos per batch, or g per pack, but the same unit should be used throughout this column. To assist the retailer with reviewing the formulation, it is useful to state what units have been used in the Comments field later in this section. |
|
Batch % |
The percentage breakdown of the Quantities in Batch. This is automatically calculated when Calculate & Create Ingredient List is clicked. |
|
Total % |
The percentage breakdown of all Declared ingredients, including adjustments for Ingredients which have been summed together (either by name or by Forced Addition) and for any water losses applied. This is automatically calculated when Calculate & Create Ingredient List is clicked. |
|
Dec |
Check the box to indicate all ingredients which must be declared in the Ingredient List. For Ingredients which are summed (either by name or by Forced Addition), only check the first occurrence of that Ingredient. The system prevents you from checking multiple occurrences of summed ingredients. For Compound Ingredients:
|
|
No. of supp |
This column displays the number of Raw Material Suppliers which have been entered for each Ingredient. Click the icon to access the Raw Material Data Entry window. Add rows to the table to represent the number of Suppliers for this ingredient. Separate entries must be added for each supplier. Enter the relevant details from each Supplier's Ingredient Specification. Clicking Ok update the number of Suppliers displayed in this column and updates the Raw Material tab with the details you have entered. The last row (Supplier) for any Ingredient may not be removed. Note: The Raw Material details may either be entered directly using the table in the Raw Materials tab or the Raw Material Data Entry window, described above and accessed by clicking the icon in the No. of supp column in the Formulation table. However, the number of Supplier rows may only be added or removed using the Raw Material Data Entry window. |
Single Ingredients, Compounds, and Components
Columns 1 and 2 in the Formulation table are used to indicate any compound ingredients and their components:
-
Use column 1 to indicate Compound Ingredients. Use a simple numbering system, for example, the first compound in the formulation = 1, second compound = 2, and so on.
-
List the Component Ingredients directly below the name of the Compound Ingredient and use column 2 to indicate the number of the compound to which they belong, for example, 1, 2, and so on, as above.
-
Nested Compounds, that is, where a Compound or Sub Recipe contains Compound Ingredients itself, will have an entry in both columns 1 and 2, for example, Compound 2a, Component of Compound 2.
-
Leave columns 1 and 2 blank for Single Ingredients which are added directly to the final batch.
Water Loss
The system enables Suppliers to demonstrate any water lost from the formulation as part of a heating process, with the effect that the water ingredient may move further down the ranking in the finished Ingredient List as it evaporates.
To achieve this, add a second entry in the recipe table for Water, with a negative quantity to demonstrate the required water loss. If the initial Water input is through a Compound ingredient, the second entry for Water should be indicated as being part of the same Compound (by placing the Compound reference in column 2), and the water-loss quantity should be placed in the Qty in premix column.
Adding Comment Rows
For ease of reading or to indicate different sub-parts to the Formulation, it may be useful to insert a blank row or a comment. Enter number sign (#) into column 1 in the relevant row. All columns in that row become disabled, except for the Ingredient column which may either be left blank, or a suitable comment or a heading for the following part of the Formulation can be entered. Any comments# rows are not subject to validation against the Ingredient Glossary and do not appear in the ingredients statement.
How to Generate the On-Pack Ingredients List
To generate an on-pack ingredients list:
-
Add sufficient rows to the Formulation table and enter all Ingredients, Compound Ingredients, all Forced Additions where required, and all Quantity information.
-
Check the Dec column to indicate all Ingredients and/or Compounds to be included in the on-pack Ingredient List.
-
Click Calculate & Create Ingredient List. The Formulation is now validated and any errors are displayed in a window. You must correct these errors and then use the Calculate & Create Ingredient List again.
-
Upon successful validation, the system-generated Ingredient List appears, for reference purposes, on the tab below the Formulation Table. The Ingredient List, in both system-generated (read-only) and editable formats, will appear on the Declarations Tab.
-
Add any additional Comments or supporting Attachments to the subtabs adjacent to the Ingredients tab.
Raw Material Tab
This tab provides a list of all Ingredients which have been entered into the Formulation table, with multiple rows for ingredients which have more than one Supplier. Additional details, such as Grade / Specification and Origin details, can be entered for each Ingredient and each Supplier.
The Raw Material details may be entered directly using the table in the Raw Materials tab or by using the Raw Material Data Entry window, accessed by clicking the icon in the No. of supp column in the Formulation table. However, Supplier rows may only be added or removed using the Raw Material Data Entry window.
Click the icon in the No. of supp column of the Formulation table to access the Raw Material Data Entry window. Add rows to the table to represent the number of Suppliers for this ingredient. Separate entries must be added for each supplier. Enter the relevant details from each Supplier's Ingredient Specification. Clicking Ok updates the Raw Material tab with the details you have entered.
Note:
The last row (Supplier) for any Ingredient may not be removed. System defaults to require one row per ingredient.Table 3-54 describes the columns.
Table 3-54 FNF Raw Material Page
| Column | Description |
|---|---|
|
Ingredient |
Name of Ingredient entered in the Formulation Table. Populated automatically with multiple rows for Ingredients which have more than one Supplier. |
|
Function |
If appropriate, select the type of ingredient from the pick-list. |
|
Trade Name |
Insert the trade name of the ingredient, if used. |
|
Grade / Specification |
Free text field which enables you to enter specific details about the nature of the Ingredient, usually obtained from the Raw Material Specification. |
|
Supplier |
Enter the name of the Raw Material supplier. |
|
Year Of Last Animal Test |
An optional field used to select the year of testing. |
|
Reason For Animal Test |
An optional field used to select the reason from a list. |
|
Country of Origin |
Click the Country icon. Use the search option to locate the required countries. Use the check boxes to select multiple countries, if necessary. |
|
Sustainability Category |
Select a sustainability category to indicate if ingredients fall into particular categories of concern regarding sustainability. The selection applies to all raw material rows for the ingredient. This column will only show if the Sustainability feature is enabled for Formulated Non Food specifications. |
|
Material Supplier Connections |
See the "Recipe and Raw Materials Section" in the Food Specification section for details. |
|
Raw Materials Info |
Displays the Yes/No option which was selected for Raw Material Info Required in the Raw Material Data Entry window. This option may only be set to No by the retailer. If set to No, the mandatory fields for that ingredient are suppressed. |
Sustainability Tab
This tab contains the Sustainability data, in a table which is automatically populated with a row for each ingredient in the Raw Materials table where a sustainability category has been assigned. The rows are presented in the order they appear in the Raw Materials table. If the ingredient has more than one supplier in the Raw Materials table, a row will be present for each supplier.
Note:
This tab only appears if the Sustainability feature is enabled for Formulated Non Food specifications.Table 3-55 describes the fields.
Table 3-55 FNF Sustainability Page
| Column | Description |
|---|---|
|
Sustainability Category |
The Sustainability Category entered in the Raw Materials table. Populated automatically by the system, with multiple rows for ingredients that have more than one Supplier. |
|
Ingredient |
Name of Ingredient entered in the Raw Materials table. Populated automatically by the system, with Ingredients that have a sustainability category assigned, with multiple rows for ingredients that have more than one Supplier. |
|
Supplier |
The name of the Supplier entered in the Raw Materials table. |
|
Breed/Latin Name/Variety |
Select the Ingredient's breed, Latin name or variety, if relevant. The available selections are filtered to just show those that are associated to the ingredient's sustainability category. |
|
Source |
Select the Ingredient's source, if relevant. The available selections are filtered to just show those that are associated to the ingredient's sustainability category. |
|
Catch Method |
Select the Ingredient's catch method, if relevant. The available selections are filtered to just show those that are associated to the ingredient's sustainability category. |
Note:
If an ingredient's sustainability category is changed in the Raw Materials table, the contents of the Breed/Latin Name/Variety, Source, and Catch Method columns will be cleared.Material Transparency Tab
This tab contains the Material Transparency tree view, a hierarchical representation of the raw materials supply chain.
Note:
This tab only appears if the Material Transparency feature is enabled for Formulated Non Food specifications.See the "Recipe and Raw Materials Section" in the Food Specification section for details.
Declarations Tab
This tab is used to manage the ingredient declarations as they appear on the product package.
Table 3-56 describes the fields.
Table 3-56 FNF Declarations Page
| Field | Description |
|---|---|
|
Pack Copy Ingredients List |
System-generated, cannot be edited. As displayed, calculated, and created on the Formulation tab. |
|
Business Language Ingredients List |
System-generated in the portal's business language, cannot be edited. As displayed, calculated, and created on the Formulation tab. |
|
Is a cosmetic ingredient list required pack |
Select Yes or No. Controls which fields then appear for capture of the ingredients list. |
|
Ingredients (Household) |
If the cosmetic ingredient list required on pack is No, this free text field appears for the entry of the ingredients list. |
|
For an ingredients datasheet visit (Detergents only) |
If the cosmetic ingredient list required on pack radio button is No, this free text field appears. Provide a website address if one is required. |
|
On-Pack Ingredients |
If the cosmetic ingredient list required on pack radio button is Yes, this field appears. It is automatically populated with the system-generated Ingredient List (English version), which can be manually edited as appropriate. Text may also be emboldened and italicized. Note: Manually edit for formatting purposes. If an ingredient name is wrong or needs changing, contact your System Administrator, representative at the retailer, or the Service Desk to report a necessary change in the master ingredient glossary. |
|
Fragrance Allergens |
If the cosmetic ingredient list required on pack radio button is Yes, this field appears. Enter details of any allergen relating to fragrances used. |
|
For a medical data sheet (medical personnel only) contacts (Supplier PO Box/Address, Phone) |
For the entry of medical data sheet contact details. Provide details if required. |
Validation of the Formulation and Raw Materials Section
Validation is applied when calculating the on-pack Ingredient List. In addition, the full Specification Validation process runs when using the Validate options from the action menu, or when attempting to progress the Specification to another status.
Validation of the Formulation and Raw Materials section performs the checks outlined below. Any errors must be resolved before the Specification can be progressed.
Validating the Formulation Tab
Validating the Formulation Tab
The following validation is performed:
-
The Ingredient column must be completed for all rows, except where a comment (#) is entered.
-
All Ingredient Names must match valid ingredient names held in the Glossary.
-
Either the Qty In premix or Batch Qty column must be completed for each Ingredient.
-
After entering the Formulation, the Calculate & Create Ingredient List button must be used and the Ingredient List successfully generated before the specification can be progressed to the next status. If the Formulation is amended, after having used the Calculate & Create Ingredient List option, the option must be used again before the specification can be progressed.
Note:
The text on the Calculate & Create Ingredient List button changes to red if the Ingredient List has not yet been successfully generated (due to errors found) or if the Ingredient List needs to be regenerated due to the Formulation being amended.Although all ingredient names are validated against the glossary, the following are not validated:
-
The names of compound ingredients.
-
If a number sign (#) is entered in column 1 to indicate a comment row.
Note:
If you cannot locate the required ingredient in the Glossary, even after considering any alternative names it might have, contact your System Administrator, representative at the retailer, or the Service Desk to arrange for a new ingredient to be added to the Glossary, or to suggest the correct ingredient they should be using. The request should be quickly reviewed and validated by the retailer who will then add approved new ingredients to the glossary.Validating the Raw Materials Tab
All mandatory fields are indicated by an asterisk (*).
Validating the Declarations Tab
The Is a cosmetic ingredient list required on pack field must be completed.
PIF Information Tab
This section details the Safety Data Sheets/Adverse Event Reporting information.
Table 3-57 describes the fields.
Table 3-57 FNF PIF Information Page
| Field | Description |
|---|---|
|
Safety Data |
|
|
Registered with National Poisons Information Service? |
Select Yes or No. |
|
Safety Data Sheet Attached? |
Select Yes or No. |
|
Address |
|
|
Country Address 1 Address 2 City County Post Code GPS latitude GPS longitude |
Enter the address details for the organization responsible for the product safety information. |
|
Product Information File |
|
|
Person Responsible |
Indicate the responsible person for the product safety information. |
|
Phone Fax Number Email Address Company Safety Assessment carried out by name |
Free text fields for the responsible person's details. |
|
Assessment Attached |
Select Yes or No. |
Validating the PIF Information Tab
Only the options for Safety Data Sheet Attached? and Registered with National Poisons Information Service are mandatory in this tab.
Additional Product Information Section
The Additional Product Information section captures all the information on sensitive materials used within the product, as well as, indicating for whom the product is suitable. This section is based on the Food specification's Allergy and Dietary Advice (D&A) section.
Table 3-58 describes the field sets.
Table 3-58 FNF Additional Product Information Page
| Field Set | Description |
|---|---|
|
Questions |
Captures the details of the presence or use of sensitive materials in the Product. In addition, where an item is Present and its Source. |
|
Source |
If the material is present, the source shows why it is present, for example, in which component. |
|
Validation Overrides |
Validation may suggest that the sensitive material is present in the product, but it is known that the implicated material is not actually problematic. The Validation Overrides table provides the formal process for overriding the system validation. |
How to Complete Responses to the Questions
Complete all responses to each question, using the Yes/No options to indicate whether the sensitive material is Present in the Product.
How to Complete the Source column in the Allergen Table
For any sensitive material which is marked as Present in Product, the Source field appears and must be completed.
Validation of Answers to Questions
For Contains questions, if Present is Yes, then the Source must be entered.
-
For Contains questions marked as Not Present in Product or Suitable for questions marked as Present, validation automatically checks the Formulation, Ingredient List, Product Name (on pack), and Secondary Descriptor for the presence of the sensitive material in question, based on a list of Intolerant substances stored against each question and controlled by the retailer's System Administrator.
-
If the validation suggests that the sensitive material is present, a terminal validation error is given (preventing the specification from being progressed to the next status) and an entry appears in the Validation Overrides table.
-
The user must either correct the response to Present in Product (which will remove the error and remove the entry from the Validation Overrides table) or, if it is known that the implicated material does not pertain to the question, the Request Override and Override Reason fields must be completed in the Validation Overrides table.
-
The retailer must complete the Approval checkbox in the Validation Overrides table before the specification can be moved to Pack Copy Sent status.
-
As an example, for a Phthalate question, the word Phthalate has been entered against the question. If the Product Name (on pack) contains the phrase "without phthalate," there will be a terminal error as the word 'Phthalate' will have been found and clearly it is not present. Completing the Override fields suppresses the terminal error.
Validation Overrides will be used infrequently.
Claim Substantiation Section
The Claim Substantiation section provides evidence to confirm any claim made for the product, or any standard to which it conforms. It consists of a single table and a Comments area.
For each claim made or standard conformed to, the following details are entered:
-
Standard or Claim description.
-
The method used to confirm the claim or standard.
-
The Test body/house or laboratory used to establish the claim.
-
Date of approval or certification.
-
Is the certificate attached to this specification.
To add more claims to the table, click Add or Insert in the table header.
Use the Comments field to add any additional information to support or qualify entries in the table.
Process Controls Section
The Process Controls section specifies the process controls which are applied during manufacturing and packing of the product. This section is based on the Food specification's Process Controls section.
Table 3-59 describes the pages.
Table 3-59 FNF Process Controls Pages
| Page | Description |
|---|---|
|
Process Steps |
Use to outline process steps, units, operations, and key control points. This rich text field enables formatting to be applied to present the process steps clearly. Highlight the text you wish to apply formatting to and click the appropriate icon from the bar at the top of the field. |
|
Critical Control Points (CCPs) |
Add sufficient rows to the table for each Critical Control Point. Complete the free text fields for Process Step, CCP No., Hazard, Control Measures, Critical Limits, Monitoring Procedures, and Corrective Actions. |
|
Quality Control Points (QCPs) |
Add sufficient rows to the table for each Quality Control Point. Complete the free text fields for Process Step, Legal/Quality Issue, Control Measures, Tolerance, Monitoring Procedures, and Corrective Actions. |
Validation of the Process Controls Section
No specific validation is applied to the Process Controls section.
Storage Section
The Storage section specifies the storage requirements of the product, including work in progress and the finished product. This section is based on the Food specification's Storage section.
Table 3-60 describes the fields.
| Field | Description |
|---|---|
|
Stability Temperature Range (°C) |
A pair of from/to numeric fields for the acceptable storage temperature range. |
|
Product life unopened |
A numeric field for the life and a pick-list for its units. |
|
Product life after opening (where applicable) |
A numeric field for the life with a pick-list for its units. |
|
Pack Coding Details |
A descriptive text field. |
This is a table of four columns followed by additional questions:
-
Table:
-
Time on test (at what intervals).
-
Storage Conditions (under what conditions did stability occur, room temperature, 40°C humidity, and so on.)
-
Tests (details of tests conducted at that interval).
-
Results (the results of the test).
-
-
Additional Fields:
-
Date when satisfactory 3 months stability results obtained (icon to select the date).
-
Date when satisfactory challenge test obtained (icon to select date).
-
This field-set contains only one free text field:
-
Transit Trials and Drop Tests Details (description of the trials carried out).
Validation of the Storage Section
There are no mandatory fields in this section.
Packaging Section
The Packaging section specifies the Packaging format and all packaging components and materials used for the product, including the retail pack and any secondary and tertiary packaging. This section is based on the Food specification's Packaging section. For more information, see the "Packaging Section" in the Food Specification section.
Validation of the Packaging Section
The Specification Validation process will run when using the Validate options from the action menu or when attempting to progress the Specification to another status. All mandatory fields are indicated by an asterisk.
Finished Product Standards Section
The Finished Product Standards (FPS) section specifies the finished product standards for the product, including Product Attributes, Physical Product and Package Standards, and Chemical and Microbiological Standards. This section is based on the Food specification's FPS section.
Products Attributes Tab
This tab contains three subtabs within it, for the product as sold, as used and for details of the benchmarks.
Table 3-61 describes the fields in the pages for the three subtabs.
Table 3-61 FNF Product Attributes Pages
| Field | Description |
|---|---|
|
Project Attributes: As Sold and As Used |
Enter all qualitative attributes for the predefined attributes (Packaging, Appearance, Aroma, Texture, and Flavour). Following are the typical classifications for Green, Amber/Yellow, and Red:
|
|
Additional Attributes: As Sold and As Used |
Use the table to enter any further attributes which are tested for this product and not mentioned in the main Product Attributes table. |
|
Benchmarks |
Pulls through the Benchmark details which are present on any linked Product Records for reference. Any amendments to this information must be made on the Product Records themselves (however the data may only be edited by retailer). |
Physical Product Tab
This tab captures the details of any physical product and package standards, such as color, viscosity, and so on.
Table 3-62 describes the fields.
Table 3-62 FNF Physical Product Page
| Field | Description |
|---|---|
|
Physical Quantitative Standards |
If physical testing is carried out, provide details. Enter sufficient rows to the table for the testing which is carried out. For each test, the fields for Minimum, Target, Maximum, Units, Method, and Frequency must be completed if a test has been entered. If sets of standards have been preconfigured, one may be selected from the Physical Standards glossary. Selecting from the glossary will overwrite the contents of the table (following a warning prompt). If standards are preconfigured in the glossary, one of them may have been designated the default set, in which case the table will be automatically populated with that standard when creating a new specification. If the table is populated with predefined values from the glossary, they may then be edited or removed, or additional rows added. If the Test column is populated, a value must be entered in all other columns. |
|
Other Physical Standards |
Provide details of any other physical standards which are applied to the product or package. This rich text field enables formatting to be applied. |
Chemical Standards Tab
This tab captures the details of any chemical standards, such as pH, moisture, % salt, % oil, and so on.
Table 3-63 describes the fields.
Table 3-63 FNF Chemical Standards Page
| Field | Description |
|---|---|
|
Chemical Quantitative Standards |
If chemical testing is carried out, provide the details. Enter sufficient rows to the table for the testing which is carried out. For each test, the fields for Minimum, Target, Maximum, Units, Method, and Frequency must be completed if a test has been entered. If sets of standards have been preconfigured, one may be selected from the Chemical Standards glossary. Selecting from the glossary will overwrite the contents of the table (following a warning prompt). If standards are preconfigured in the glossary, one of them may have been designated the default set, in which case the table will be automatically populated with that standard when creating a new specification. If the table is populated with predefined values from the glossary, they may then be edited or removed, or additional rows added. If the Parameter column is populated, a value must be entered in all other columns. |
|
Other Chemical Standards |
Provide the details of any other chemical standards which are applied to the product or package. This rich text field enables formatting to be applied. |
Microbiological Standards Tab
This tab captures the details of any Microbiological standards.
Table 3-64 describes the fields.
Table 3-64 FNF Microbiological Standards Page
| Field | Description |
|---|---|
|
Microbiological Classification |
A Retailer may configure standard microbiological standards for certain product types, for example, all pre-packaged sandwiches of a particular type may have a standard microbiological specification. A classification may be selected here. This then results in an additional table of standards being displayed where the Test, Target, Maximum, and Units are predefined and read-only and the respective Method and Frequency is then completed by the Supplier. |
|
Tests |
If microbiological testing is carried out, provide the details. Enter sufficient rows to the table for the testing which is carried out. For each test, the fields for Target, Maximum, Units, Method, and Frequency must be completed. |
|
Other Microbiological Standards |
Provide the details of any other microbiological standards which are applied to the product or package. |
Validation of the Finished Product Standards Section
The Specification Validation process will run when using the Validate options from the action menu, or when attempting to progress the Specification to another status. All mandatory fields are indicated by an asterisk.
Other Labelling Copy Section
The Other Labelling Copy (OLC) section captures all on-pack labelling requirements for the product, which have not already been provided in the Formulation and Raw Materials section. Any details entered in this section will be passed through to the Pack Copy file, so fields should be left blank if they are not required. This section is based on the Food specification's OLC section.
Products Tab
This tab captures details of the product names and branding details.
Table 3-65 describes the fields.
| Field | Description |
|---|---|
|
Brand and Sub Brand |
Brand details pulled from the Main Details section of the Specification. This field is read-only so any amendments must be made in that section. |
|
Product Range |
Free text to enter the range description exactly as it will appear on the pack. |
|
Main Product Title |
Free text field to enter the name of the product exactly as it will appear on the pack. |
|
Additional Front of Pack Copy |
Free text field to enter any additional fanciful marketing product descriptor exactly as it will appear on the pack. |
|
Product Legal Title |
Free text field to enter the legal/regulated name of the product exactly as it will appear on the pack. |
|
Back of pack marketing copy |
Free text field to enter the marketing copy exactly as it will appear on the pack. |
Quantity Tab
This tab captures the on-pack quantity declaration for the product.
Table 3-66 describes the fields.
| Field | Description |
|---|---|
|
Declared Quantity Type |
Available options are Fixed quantity and No declared quantity. If Fixed quantity is selected, the following fields are shown. Otherwise, the fields are hidden. |
|
Declared Quantity |
Enter the actual quantity as it is to appear on the pack. This is a mandatory field. |
|
Location |
From the pick-list, indicate whether the declared quantity is required on pack and its location. |
|
e-mark Required (min height 3mm) |
Single checkbox to indicate if an e-mark is required. |
|
Print Height |
Three pick-lists are used to specify the print height required for the net quantity. Select one of the pick-list options to calculate the print height. |
|
Brimful Container Capacity (Aerosols) |
Numeric field. |
Flashes and Logos Tab
This tab captures any on-pack icons, logos, claims, flashes, and statements. Complete only the fields which are required on pack for this product.
Table 3-67 describes the fields.
Table 3-67 FNF Flashes and Logos Page
| Field | Description |
|---|---|
|
Flashes |
|
|
Flashes |
Select a statement from the list. The selected statement appears in the table. |
|
Duration (weeks) |
Numeric field. |
|
Logos |
|
|
Icon |
Select a statement from the list. The selected statement appears in the table. |
|
Supporting Text for Declarations |
Text field for information that supports the selected icons and flashes. |
|
Use on Pack |
Indicates whether this is required on pack and its location. |
Directions Tab
This tab captures details on how the product should be used. Complete only the fields which are required on the pack for this product.
Table 3-68 describes the fields.
Storage and Warnings Tab
This tab captures any on-pack storage and warning information. Complete only the fields which are required on the pack for this product.
Table 3-69 describes the fields.
Table 3-69 FNF Storage and Warnings Page
| Field | Description |
|---|---|
|
Storage Phrase |
Select a statement from the list. |
|
Date Coding |
Select the format on pack. |
|
Is Period After Opening symbol required |
Options are Yes and No. If Yes is selected the Period (Months) is also shown, which is a mandatory numeric field. |
|
Warnings (Household) |
Select a statement from the list. |
|
Warnings (Cosmetics) |
Select a statement from the list. |
|
Warnings (Pharmaceuticals) |
Select a statement from the list. |
|
CHIP Panel |
|
|
Is a CHIP panel required on a product |
Options are Yes and No. If Yes is selected, the following fields are shown. Otherwise, the fields are hidden. |
|
Product Name |
Main Product Title pulled from the Products tab. This field is read-only, so any amendments must be made in that tab. |
|
Contains |
Provide the name of any hazardous material present. |
|
Hazard |
Select a statement from the list. |
|
Risk and Safety Phrases |
Select a statement from the list. |
|
Produced In |
Select a country from the list. |
|
Quantity |
Declared Quantity pulled from the Quantities tab. This field is read-only, so any amendments must be made in that tab. |
Other Back Of Pack Tab
This tab captures any remaining information which should appear on the pack.
Table 3-70 describes the fields.
Table 3-70 FNF Other Back Of Pack Page
| Field | Description |
|---|---|
|
Environmental Phrases |
Select a statement from the list. |
|
Environmental Logos |
Select a statement from the list. |
|
Symbols |
Select a statement from the list. |
|
Additional Information |
Free text field to enter any additional information. |
|
Price Box |
Option defaults to not required. Change to an appropriate option if a price box is required on the pack. |
|
Standard Price |
Free text to enter the price details, if applicable. |
|
Launch Promo Price |
Free text to enter the price details, if applicable. |
|
Country of Origin Statements |
From the Statement pick-list, choose the applicable on-pack statement. Two statement options are available, to accommodate products which require them. From the Country pick-lists, choose the applicable countries. Multiple countries can be selected for each statement. At least one statement/country must be specified. |
|
For |
Select the ownership text required by the retailer. |
|
Copyright Year |
Enter the corresponding copyright year, if applicable. |
|
Supplier/Site code on Pack |
Options defaults to No. Use to indicate whether the supplier and/or site codes should be included on the pack. |
|
Product Licence Holder and Number (Medicines) |
Free text field to enter licence details for medicines. |
|
MAPP or HSE No. PCS No. (for ROI) |
Free text fields to enter health and safety references as applicable to the product and its market area. |
|
EAN/Barcode Shipping Case Code |
The product and shipping case barcode, EAN or UPC codes. The list may be automatically populated when the specification is linked to its Product Record. To add multiple rows to the table select Add, or check the box next to a row in the table and use Delete to remove it. |
Non Copy Information Tab
This tab provides instructions and information to the design team regarding printing and design requirements. The information completed in this tab is not intended to appear on the pack itself.
Table 3-71 describes the fields.
Table 3-71 FNF Non Copy Information Page
| Field | Description |
|---|---|
|
Details |
|
|
Pack Copy to be Forwarded To |
This provides the email address to whom the Pack Copy files will be sent for this Specification. Select the required name from the list. |
|
Design Comments |
Free text field to enter any additional instructions or comments to assist the design team. |
|
Reason for Issue |
Free text field to enter details of the reason for generating the Pack Copy files. Use to assist the design team in locating any amendments from a previous version, for example. |
|
Details of other documents to be sent separately |
Free text field to enter details of any other printed materials which form part of the product package. May be sent to the design team separately in the Pack Copy files, such as style guides, sample design package, information leaflets, and so on. |
|
CAD Ref. |
Free text field to enter any additional design references. |
|
Cutter Guide Attachments |
This is a special area to attach any additional documents which need to be sent to the design team at the same time as the Pack Copy files. The attachments located here are automatically included along with the specification's Pack Copy file when it is generated and sent in email to the design team. |
|
Packaging Design Date |
Select the calendar icon to enter the planned date for completion of the packaging design. |
|
Film to Printer Date |
Select the calendar icon to enter the planned date for the film to be delivered to the printer. |
|
Pack Copy Status |
This text field can be used to manually record the progress of the creation of the specification's Pack Copy file. |
|
Will Specified Board and Inks be used? |
Indicate the use of master boards in the print process. If No is selected, provide a Reason in the adjacent field. |
|
Primary Pack Format |
Free text field to list the items that make up the primary packaging format. |
|
Photography - confirmation of when products will be ready to shoot |
Free text field to enter details of when the products will be available for photographing for the pack design. |
|
Shelf Ready Packaging - is product in SRP? (if not give reason) |
Free text field to enter details of the product's shelf ready packaging, or a reason if there is none. |
|
Current/Proposed Format |
Free text field to enter details of the current or proposed format used in the packaging design. |
|
Current/Proposed Material |
Free text field to enter details of the current or proposed material used in the packaging design. |
|
Printers |
|
|
Add sufficient rows to the table to provide details for each of the printers that are involved in printing the packaging for this product. Multiple rows may be added per printer if the printer handles more than one printed component. |
|
|
Name |
Free text field to enter the name of the printer company. This is a mandatory field. |
|
Contact Name |
Free text field to enter the name of the contact at the printer company for this packaging. This is a mandatory field. |
|
Email, Phone, and Fax |
Free text fields to enter the email, phone, and fax details for the printer company. Email and Phone are mandatory fields. |
|
Address details |
Free text field to enter the address of the printer company. Country is a mandatory field. |
|
Packaging Component |
Free text field to enter the name of the printed component. |
|
Print Process |
Free text field to enter the print process used for this printed component. This is a mandatory field. |
|
Print Substrate |
Free text field to enter the type of print substrate for this printed component. This is a mandatory field. |
|
Packaging Format |
Free text field to enter the packaging format for this printed component. This is a mandatory field. |
|
Colours |
Free text field to enter the colors used in the print process for this printed component. This is a mandatory field. |
|
Approval Details |
|
|
Retailer Approval Name Retailer Approval Date Retailer Approval Position Pack Copy Issue |
These fields are read-only and are system-generated when the specification's Pack Copy files are generated. |
Validation of the Other Labelling Copy Section
The Specification Validation process runs when using the Validate options from the action menu, or when attempting to progress the Specification to another status. All mandatory fields are indicated by an asterisk.
Advanced Packaging Section
The Advanced Packaging section provides an alternative to the Packaging Section allowing for the collection of more packaging data than the standard Packaging Section. Included are reporting capabilities and recycling information handling. This section is based on the Food specification's Advanced Packaging section. For more information, see the "Advanced Packaging Section" in the Food Specification section.
Project Links Section
This section is defined as per the Food Specification. For more information, see the "Project Links Section" description for the Food Specification section.
Custom Fields Section
This section is defined as per the Food Specification. For more information, see the "Custom Fields Section" description for the Food Specification section.
Attachments Section
This section is defined as per the Food Specification. For more information, see the "Attachments Section" description for the Food Specification section.
Change History Section
This section is defined as per the Food Specification.
Constructed Non Food Specification
The Constructed Non Food (CNF) specification contains the following sections:
The workflow of the CNF specification and general specification management framework functionality, such as the relationship to Product Records and Pack Copy files and management of multi specifications, is as described for the Food specification.
The Header and Main Details section are defined per the Food specification. The core default Specification Type is Constructed Non Food. The Specification Name is mandatory for a new specification to be saved.
Main Details Section
For more information, see the "Main Details Section" description for the Food Specification section.
Components Section
The Components section is where all the components of the product are described in detail with their quantities, grades, suppliers, countries of origin, and specific details for paper, wood, and fabric components if part of the product.
Quantities Tab
This table lists all the component parts of the product with their quantities.
To add new rows to the table, click Add or Insert. Add as many rows as needed. The following columns are shown:
-
Item - The items that make up the product. For example, Table and Chair could be items for a BBQ table and chair set; a kitchen knife would simply be Knife.
-
Component - The components that make up the item. For example, Top, Legs, and Fixings for a self-assembly table.
-
Material - What the component is made from.
-
% Material in Component - Very often, 100%. For example, brass rivets in a knife handle, but a veneered table top could be 95% pine.
-
Component Quantity in Item - Typically, the weight of the component in the item (not in the product).
-
Quantity of Item in Product (g) - The weight in grams of the component in the overall product.
-
% of Item in product - Calculated by clicking the Calculate % of Item in Product tab at the top of the table. Calculates the Quantity of Item in Product (g) as a percentage of the sum of all quantities. Non-editable.
-
Number in Product - The quantity of each component in the product, for example, four table legs.
-
Number of Suppliers - The number of suppliers of each component. Click the button to add/remove suppliers. A dialog box is presented to add more details.
-
Fabric, Formulated, Paper & Wood - A pick-list to denote that this component is made from Fabric, Paper, or Wood, or is a Formulated component, such as, the moisturizer component of a moisturizing wipe. More details on these components are added in the Formulations, Paper and Wood Details, or Fabrics tabs if the component is specified as one of these four types. Leave blank if the component is not made of any of these options.
Sufficient information to define the product adequately in terms of materials and quantities is all that is required, without excessive detail, so not all columns need to be completed for each component.
Below the table are the following three fields:
-
Comments - List anything that needs further explanation regarding the components table or items within it.
-
Is a Components list required on pack? - If set to Yes, the Ingredients field is output to the Pack Copy file, and an Ingredients field displays below.
-
Ingredients - A list of ingredients or components that make up the product. This field is hidden if the answer above is No. Can be edited by a user to display the correct wording to display on pack.
Details Tab
This table stores the details of all the component parts of the product.
The table is of fixed size and has a row for each supplier of each component in the Quantities table, with the Item, Component, and Material fields copied from the Quantities table. The following columns are shown:
-
Item, Component, Material - Copied from the Quantities table.
-
Ref - The suppliers' reference for this component to assist them in identifying exactly which component is in the product.
-
Specification - Any specification the component conforms to.
-
Finish/color - Any finish or color applied to the component.
-
Approval Standard - Any standard the component conforms to.
-
Test House - If the component is tested, provide details of testing here.
-
Suppliers - Names of the suppliers of this component. This is a mandatory field.
-
Country of Origin - Select the country of origin of the component. This is a mandatory field.
Formulations Tab
This table stores details of any formulated part of the product. Any formulated component would be minor; otherwise, a Formulated Non Food spec would be used with additional sections for Constructed Non Food.
If the product contains a component that has a Formulation, it can be described fully here. For example, a hanging basket with compost, the compost being the formulated component. The following columns are shown:
-
Item and Component - Use the pick-lists to select from those Components in the Quantities table marked as Formulation in the far right column. Both are mandatory.
-
Ingredients - Add rows to the table for each ingredient, and enter the name. This field is a mandatory field.
-
Trade Name - Complete as appropriate.
-
Batch Quantity and Units - Complete as appropriate.
-
% w/w - percentage by weight - Complete as appropriate.
The following field appears below the table:
-
Registered with the National Poisons Information Service - Complete as appropriate.
Paper and Wood Details Tab
This table stores details of any wood or paper used in the product. The following columns are shown:
-
Item and Component - Use the pick-lists to select from those Components in the Quantities table marked as Wood or Paper in the far right column. Both are mandatory.
-
Wood Species Used - Provide in scientific name format (genus, species).
-
Country of Origin of Wood - Select the country of origin of the wood. This is a mandatory field.
-
Name of Saw or Paper Mill - Complete as appropriate.
-
Weight of wood species in component - Complete as appropriate.
-
% of wood species in component - Complete as appropriate.
Fabrics Tab
This table stores details of any fabrics used in the product. The following columns are shown:
-
Item and Component - Use the pick-lists to select from those Components in the Quantities table marked as Fabric in the far right column. Both are mandatory.
-
Features - Select from the pick-list.
-
Specification - Mandatory field to provide some detail on the features.
Additional Product Information Section
The Additional Product Information section captures all the information on sensitive materials used within the product, as well as, indicating for whom the product is suitable. This section is defined per the equivalent section in the Formulated Non Food specification. For more information, see the "Additional Product Information Section" description for the Formulated Non Food Specification section.
Product Approval Requirements Section
The Product Approval Requirements section records the details of any standards the product meets.
The table shows the testing the product conforms to, with details of any certificates or lab reports. To add rows to the table, click Add or Insert. The following columns are shown:
-
Standard - A pick-list of standards to which non-food products may be tested.
-
Test Lab / Competent Body - Enter information on the laboratory or testing body that performed the analysis.
-
Date of Approval or Certification - Select the calendar icon and choose the date.
-
Pass / Fail - Radio buttons to indicate if it is complies with the standard.
-
Comments - Further detail about testing report numbers and so on. Any test reports should be attached in the Attachments section at the bottom of the section.
-
Red Seal Approval Date - Select the calendar icon and choose the date.
-
Product Risk - Select from the options, such as High, Medium, or Low.
-
Is a test report required? - Complete as appropriate.
-
CE mark required - Complete as appropriate.
-
WEEE mark required - Complete as appropriate.
-
Is the product suitable for food use? - Complete as appropriate.
-
Are assembly/user instructions required? - Complete as appropriate.
-
Product weight without packaging (kg) - Provide the weight in kg (numeric).
-
Product Dimensions - assembled (cm) - Enter the Height, Width, and Depth (numeric).
-
How is the product delivered? - Select from the options, such as, Fully Assembled, Partially Assembled, or Component Form.
-
Product Produced in - Select one or more countries as appropriate.
-
Product Packed in - Select one or more countries as appropriate.
Process Controls Section
The Process Controls section specifies the process controls which are applied during manufacturing and packing of the product. This section is based on the Food specification's Process Controls section, but has a single combined QCP/CCP table and an additional table for in-house inspection details.
Table 3-72 describes how to use the process controls shown in Figure 3-92.
Table 3-72 CNF Process Controls Page
| Process Control | Description |
|---|---|
|
Process Steps |
Use to outline process steps, units, operations, and key control points. This rich text field enables formatting to be applied to present the process steps clearly. Highlight the text you wish to apply formatting to and click the appropriate icon from the bar at the top of the field. |
|
Critical Control Points (CCPs) and Quality Control Points (QCPs) |
Add sufficient rows to the table for each Critical Control Point and Quality Control Point, using the QCP/CCP pick-list to select the appropriate type. Complete the free text fields for Process Step, Hazard, Control Measures, Critical Limits, Method, Frequency, and Responsibility / Comments. |
|
Acceptable Quality Limits |
Use the three numeric fields to record the Acceptable Quality Limits (AQLs) for the product, and use the table to record the standards for the AQLs. |
Validation of the Process Control Section
No specific validation is applied to the Process Controls section.
Batch Coding Section
The Batch Coding section is used to describe how the product is coded.
This page has the following fields:
-
Is the product Batch Coded? - Select Yes or No.
-
Where - Select from the following options:
-
On the product
-
On the primary pack
-
On the transit pack
This is a multiple selection. For each one selected, additional fields appear asking where it is coded and its format. Deselecting hides the fields.
-
-
Where is the product batch coded and What format is used - These are mandatory fields if the On the product is selected for the Where field.
-
Where is the primary package batch coded and What format is used - These are mandatory fields if the On the primary pack is selected for the Where field.
-
Where is the transit pack batch coded and What format is used - These are mandatory fields if the On the transit pack is selected for the Where field.
-
Additional Comments
Post Launch Information Section
The Post Launch Information section is used to document reports and details of product inspections following production. This section is intended to be used after production has commenced. The section remains editable when the specification is at Active status; attachments may only be attached directly to this section while the specification is at Active status.
This page has the following fields:
-
Gold seal date approval - The date of approval of a referenced production sample, editable to retailer users only.
-
Inspection Reports:
This is a table to list the various inspection reports that may exist:
-
Inspection Number - Record any relevant reference number for the inspection, such as the PO number for the product inspected.
-
Report Number - Record any relevant reference number for the report issued.
-
Result - Brief information, such as, Accept, Pending, or Reject.
-
Date of Inspection - Click the calendar icon and select the date.
-
Accept/Reject - Editable to retailer users only.
-
Date - Date of accept/reject. Editable to retailer users only.
-
Link to Attachments - Click the icon to see all attachments linked from this area. All attachments should be uploaded in the attachments section at the bottom of the page.
-
Comments
-
-
Comments:
An area to add comments about inspections. Once comments are entered, they become read only and are not editable and cannot be deleted. This creates a history of comments.
-
Attachments Post Launch Only:
This is a special attachment manager to allow attachments to be added after the specification has been made active. Attachments may only be added or edited here if the specification is at the status of Active, and will be read-only at any status beyond that (that is, Delisted, Off Range, or Superseded). Attachments added here are not visible in the main attachments section; attachments made to the main attachments section are not visible here.
Packaging Section
The Packaging section specifies the Packaging format and all packaging components and materials used for the product, including the retail pack and any secondary and tertiary packaging. This section is defined per the equivalent section in the Formulated Non Food specification. For more information, see the "Packaging Section" description for the Formulated Non Food Specification section.
Finished Product Standards Section
The Finished Product Standards (FPS) section specifies the finished product standards for the product, including Product Attributes, Physical Product, and Package Standards, and Chemical and Microbiological Standards. This is an optional section. This section is defined as per the equivalent in the Formulated Non Food Specification. For more information, see the "Finished Product Standards Section" description for the Formulated Non Food Specification section.
Other Labelling Copy Section
The Other Labelling Copy (OLC) section captures all on-pack labelling requirements for the product, which have not already been provided in the Components section. Any details entered in this section are passed through to the Pack Copy file, so fields should be left blank if they are not required. This is an optional section. This section is based on the Food specification's OLC section.
Products Tab
This tab captures details of the product names and branding details.
Table 3-73 describes the fields.
Table 3-73 CNF Other Labelling Copy Products Page
| Field | Description |
|---|---|
|
Brand |
Brand details pulled from the Main Details section of the Specification. This field is read-only, so any amendments must be made in that section. |
|
Sub Brand |
Sub Brand details pulled from the Main Details section of the Specification. This field is read-only, so any amendments must be made in that section. |
|
Product Range |
Free text field to enter the range description exactly as it will appear on the pack. |
|
Main Product Title |
Free text field to enter the name of the product exactly as it will appear on the pack. |
|
Additional Front of Pack Copy |
Free text field to enter any additional fanciful marketing product descriptor exactly as it will appear on the pack. |
|
Product Legal Title |
Free text field to enter the legal/regulated name of the product exactly as it will appear on the pack. |
|
Back of pack marketing copy |
Free text field to enter the marketing copy exactly as it will appear on the pack. |
Quantity Tab
This tab captures the on-pack quantity declaration for the product.
Table 3-74 describes the fields
Table 3-74 CNF Other Labelling Copy Quantity Page
| Field | Description |
|---|---|
|
Declared Quantity Type |
Available options are Fixed quantity and No declared quantity. If Fixed quantity is selected, the following fields are shown. Otherwise, they are hidden. |
|
Declared Quantity |
Enter the actual quantity as it is to appear on the pack. This is a mandatory field. |
|
Location |
From the drop-down list, indicate whether the declared quantity is required on the pack and its location. |
|
e-mark Required (min height 3mm) |
Single checkbox to indicate if an e-mark is required. |
|
Print Height |
Three drop-down lists used to specify the print height required for the net quantity. Select one of the options to calculate the print height. |
Flashes and Logos Tab
This tab captures any on-pack icons, logos, claims, flashes, and statements. Complete only the fields which are required on the pack for this product.
Table 3-75 describes the fields.
Table 3-75 CNF OLC Flashes and Logos Page
| Field | Description |
|---|---|
|
Flashes |
Select a statement from the list. The selected statement will appear in the table below. |
|
Duration (weeks) |
Numeric field. |
|
Logos section |
|
|
Icon |
Select a statement from the list. The selected statement will appear in the table. |
|
Supporting Text for Declarations |
Text field for information that supports the selected icons and flashes. |
|
Use on Pack |
Indicates whether this is required on the pack and its location. |
Directions Tab
This tab captures details on how the product should be used. Complete only the fields which are required on the pack for this product.
Table 3-76 describes the fields.
Storage and Warnings Tab
This tab captures any on-pack storage and warning information. Complete only the fields which are required on the pack for this product.
Table 3-77 describes the fields.
Table 3-77 CNF OLC Storage and Warnings Page
| Field | Description |
|---|---|
|
Storage Phrase |
Select a statement from the list. |
|
Date Coding |
Select the format on pack. |
|
Warnings |
Select a statement from the list. |
|
CHIP Panel |
|
|
Is a CHIP panel required on a product |
Available options are Yes and No. If Yes is selected, the following fields are shown. Otherwise, they are hidden. |
|
Product Name |
Main Product Title pulled from the Products tab. This field is read-only so any amendments must be made in that tab. |
|
Contains |
Provide the name of any hazardous material present. |
|
Hazard |
Select a statement from the list. |
|
Risk and Safety Phrases |
Select a statement from the list. |
|
Produced In |
Select a country from the list. |
|
Quantity |
Declared Quantity pulled from the Quantities tab. This field is read-only so any amendments must be made in that tab. |
Other Back Of Pack Tab
This tab captures any remaining information which should appear on the pack.
Table 3-78 describes the fields.
Table 3-78 CNF OLC Other Back Of Pack Page
| Field | Description |
|---|---|
|
Environmental Phrases |
Select a statement from the list. |
|
Environmental Logos |
Select a statement from the list. |
|
Symbols |
Select a statement from the list. |
|
Additional Information |
Free text field to enter any additional information. |
|
Price Box |
Default is not required. Select an appropriate option if a price box is required on the pack. |
|
Standard Price |
Free text field to enter the price details if applicable. |
|
Launch Promo Price |
Free text field to enter the price details if applicable. |
|
Country of Origin Statements |
From the Statement drop-down list, choose the applicable on-pack statement. Two statement options are available to accommodate products which require them. From the Country lists, choose the applicable countries. Multiple countries can be selected for each statement. At least one statement/country must be specified. |
|
For and Copyright Year |
Select the ownership text required by the retailer, along with the corresponding copyright year if applicable. |
|
Supplier/Site code on pack |
Defaults to No. Use to indicate whether the supplier and/or site codes should be included on the pack. |
|
Product Licence Holder and Number (Medicines) |
Free text field to enter medicines and licence details. |
|
EAN/Barcode and Shipping Case Code |
These fields contain the product and shipping case barcodes, EAN or UPC codes. The list may be automatically populated when the specification is linked to its Product Record. To add multiple rows to the table, click Add. To remove a row, check the box next to the row in the table and click Delete. |
Non Copy Information Tab
This tab provides instructions and information to the design team regarding printing and design requirements. The information completed in this tab is not intended to appear on the pack itself.
Table 3-79 describes the fields.
Table 3-79 CNF OLC Non Copy Information Page
| Field | Description |
|---|---|
|
Details |
|
|
Pack Copy to be Forwarded To |
This provides the email address to whom the Pack Copy files will be sent for this Specification. Select the required name from the list. |
|
Design Comments |
Free text field to enter any additional instructions or comments to assist the design team. |
|
Reason for Issue |
Free text field to enter details of the reason for generating the Pack Copy files. For example, this information can assist the design team in locating any amendments from a previous version. |
|
Details of other documents to be sent separately |
Free text field to enter details of any other printed materials which form part of the product package. These may be sent to the design team separately to the Pack Copy files, such as style guides, sample design package, information leaflets, and so on. |
|
CAD Ref. |
Free text field to enter any additional design references. |
|
Cutter Guide Attachments |
This is a special area to attach any additional documents which need to be sent to the design team at the same time as the Pack Copy files. The attachments located here are automatically included along with the specification's Pack Copy file when it is generated and sent in email to the design team. |
|
Packaging Design Date |
Click the calendar icon to select the planned date for completion of the packaging design. |
|
Film to Printer Date |
Click the calendar icon to select the planned date for the film to be delivered to the printer. |
|
Pack Copy Status |
This text field can be used to manually record the progress of the creation of the specification's Pack Copy file. |
|
Will Specified Board and Inks be Used? |
Indicates the use of master boards in the print process. If No is selected, also provide a Reason in the adjacent field. |
|
Primary Pack Format |
Free text field to list the items that make up the primary packaging format. |
|
Photography - confirmation of when products will be ready to shoot |
Free text field to enter details of when the products will be available for photographing the pack design. |
|
Shelf Ready Packaging - is product in SRP? (if not give reason) |
Free text field to enter details of the product's shelf ready packaging or a reason if there is none. |
|
Current/Proposed Format |
Free text to enter details of the current or proposed format used in the packaging design. |
|
Current/Proposed Material |
Free text to enter details of the current or proposed material used in the packaging design. |
|
Printers Add sufficient rows to the table to provide details for each of the printers that are involved in printing the packaging for this product. Multiple rows may be added per printer if the printers handle more than one printed component. |
|
|
Name |
Free text field to enter the name of the printer company. This is a mandatory field. |
|
Contact Name |
Free text field to enter the name of the contact at the printer company for this packaging. This is a mandatory field. |
|
Email, Phone, and Fax |
Free text fields to enter the email, phone, and fax details for the printer company. Email and Phone are mandatory fields. |
|
Address details |
Free text field to enter the address of the printer company. Country is a mandatory field. |
|
Packaging Component |
Free text field to enter the name of the printed component. |
|
Print Process |
Free text field to enter the print process used for this printed component. This is a mandatory field. |
|
Print Substrate |
Free text field to enter the type of print substrate for this printed component. This is a mandatory field. |
|
Packaging Format |
Free text field to enter the packaging format for this printed component. This is a mandatory field. |
|
Colours |
Free text to enter the colors used in the print process for this printed component. This is a mandatory field. |
|
Approval Details |
|
|
Retailer Approval Name Retailer Approval Date Retailer Approval Position Pack Copy Issue |
These fields are read-only and system-generated when the specification's Pack Copy files are generated. |
Advanced Packaging Section
The Advanced Packaging section provides an alternative to the Packaging Section allowing for the collection of more packaging data than the standard Packaging Section. Included are reporting capabilities and recycling information handling. This section is based on the Food specification's Advanced Packaging section. For more information, see the "Advanced Packaging Section" in the Food Specification section.
Project Links Section
This section is defined as per the Food Specification. For more information, see the "Project Links Section" description for the Food Specification section.
Custom Fields Section
This section is defined as per the Food Specification. For more information, see the "Custom Fields Section" description for the Food Specification section.
Attachments Section
This section is defined as per the Food Specification. For more information, see the "Attachments Section" description for the Food Specification section.
Change History Section
This section is defined as per the Food Specification.
BWS Specification
The BWS specification is specifically designed for Beers, Wines, Spirits, and related alcoholic beverages. The majority of the sections are the same as those sections used within the Food Specification.
The following sections are contained in the BWS specification:
All these sections are the same as the equivalent sections within the Food Specification, with the exception of the Product Characterisation and Composition section, which is specific for BWS products.
The Other Labelling Copy Section is the same as the Food Specification Other Labelling Copy except that it does not have the Country of Origin fields as these are replaced by the Country of Origin fields in the Product Characterisation and Composition section. The Food Specification's Nutrition section may be optionally added, if required.
The following section describes the details of the Product Characterisation and Composition sections. For all other sections, refer to "Food Specification".
Product Characterisation and Composition Section
This section is made up of three tabs or pages:
Product Characterisation
The fields on this tab are all used within the Pack Copy file that is produced by the specification. Table 3-80 describes the fields.
Table 3-80 BWS Specification Product Characterisation Page
| Field | Description |
|---|---|
|
Supplier Lot ID |
Free text field for the lot ID to be pre-printed on labels. |
|
Use on Pack |
Fields with this label are used alongside each of the Pack Copy fields in this section. It is used to tell the artwork designer where the associated field data is to be printed. |
|
Geographical Identification |
Free text field used for any geographical identification that is to be printed on the label. |
|
Alcohol % |
Numeric only field used to declare the Alcohol content on the label. |
|
Components Internationally Procured |
Free text field used for any declaration required regarding internationally-procured components. |
|
Lot Volume |
Free text field used for a declaration of the volume of the lot. |
|
Country of Origin of Country of Origin |
These two fields are used to define the wording of a country of origin statement of the type: Country of Origin of Spirit: USA |
|
Country of Origin Statement |
This field is used to build complex Country of Origin Statements that are required, but that have the basic structure predefined. The field is used by selecting a base statement where additional text may be added in text boxes that are included or Countries may be selected where {c} appears within the text. |
|
Age / Vintage |
This field is used for the create age or vintage statements using a predefined structure. The field is used by selecting a base statement where additional text may be added in any text boxes that are included. |
|
Additional Information to Use on Pack |
This field is used for other statements using a predefined structure. The field is used by selecting a base statement where additional text may be added in any text boxes that are included. |
Product Composition
This section is used to define the composition of the product, with a simple table to list the ingredients used and their properties and percentages. The system does not do any calculations or validations on the composition such as the Food Recipe, but is used simply to record the details. Above the composition table, there is a single drop-down field to define the Blend Status.
Table 3-81 describes the columns in the Composition table.
Table 3-81 BWS Specification Product Composition Page Columns
| Column | Description |
|---|---|
|
Ingredient |
The ingredient names are selected from a glossary of ingredients. Clicking the selector opens a pop-up box where the different ingredient names are listed by ingredient type. The search box may be used to search for a specific ingredient. |
|
Variety |
Drop-down selector used to define the variety of the ingredient that is chosen. |
|
% in Product |
Free text field used to define the percentage of the chosen ingredient in the product. |
|
Region % |
Free text field used to specify the percentage of specific regions from which the chosen ingredient is from, if relevant. |
|
Vintage % |
Free text field used to specify the percentage of specific vintages from which the chosen ingredient is from, if relevant. |
|
Country |
Multi-select field used to specify the countries from which the ingredient is sourced. |
The Composition table is followed by additional fields that are used on the Pack Copy. Table 3-82 describes these fields.
Table 3-82 BWS Specification Product Composition Page Additional Fields
| Field | Description |
|---|---|
|
Clarification/Stabilisation/Filtration Aids |
Free text field used to define any such aids that have been used in the production that are required to be declared. |
|
Additives and Substances Used in Production |
Free text field used to define any additives or substances used in production that are to be declared. |
|
Maturation/Oak Treatments (duration and vessels) |
Free text field used to define any maturation or treatments used that are to be declared on the label. |
|
Comments |
Free text field used to add any additional comments required regarding the Product Composition. |
Custom Fields
A Retailer may add some additional Custom fields that are required for the Product Characterisation and Composition section. These Custom fields will appear in this tab. If no Custom fields are set up, the tab is not seen.
Advanced Packaging Section
The Advanced Packaging section provides an alternative to the Packaging Section allowing for the collection of more packaging data than the standard Packaging Section. Included are reporting capabilities and recycling information handling. This section is based on the Food specification's Advanced Packaging section. For more information, see the "Advanced Packaging Section" in the Food Specification section.