Sample Management
This ERD (see the following figure) illustrates how product samples can be tracked in inventory. Inventory is for a particular employee and for a specified period. All transactions involving samples such as disbursement, shipments, and sample orders can be tracked and each active inventory period can be reconciled after a physical inventory count.
Use of samples for product promotion by pharmaceutical companies around the world is governed by local country legislation. The Life Science Sampling, Sample Management and Compliance feature details requirements for sample management and compliance processes in a pharmaceutical company to ensure that the company’s processes comply with regulations. Sample Audit and Compliance Administration functionality enables companies to adhere to government guidelines.
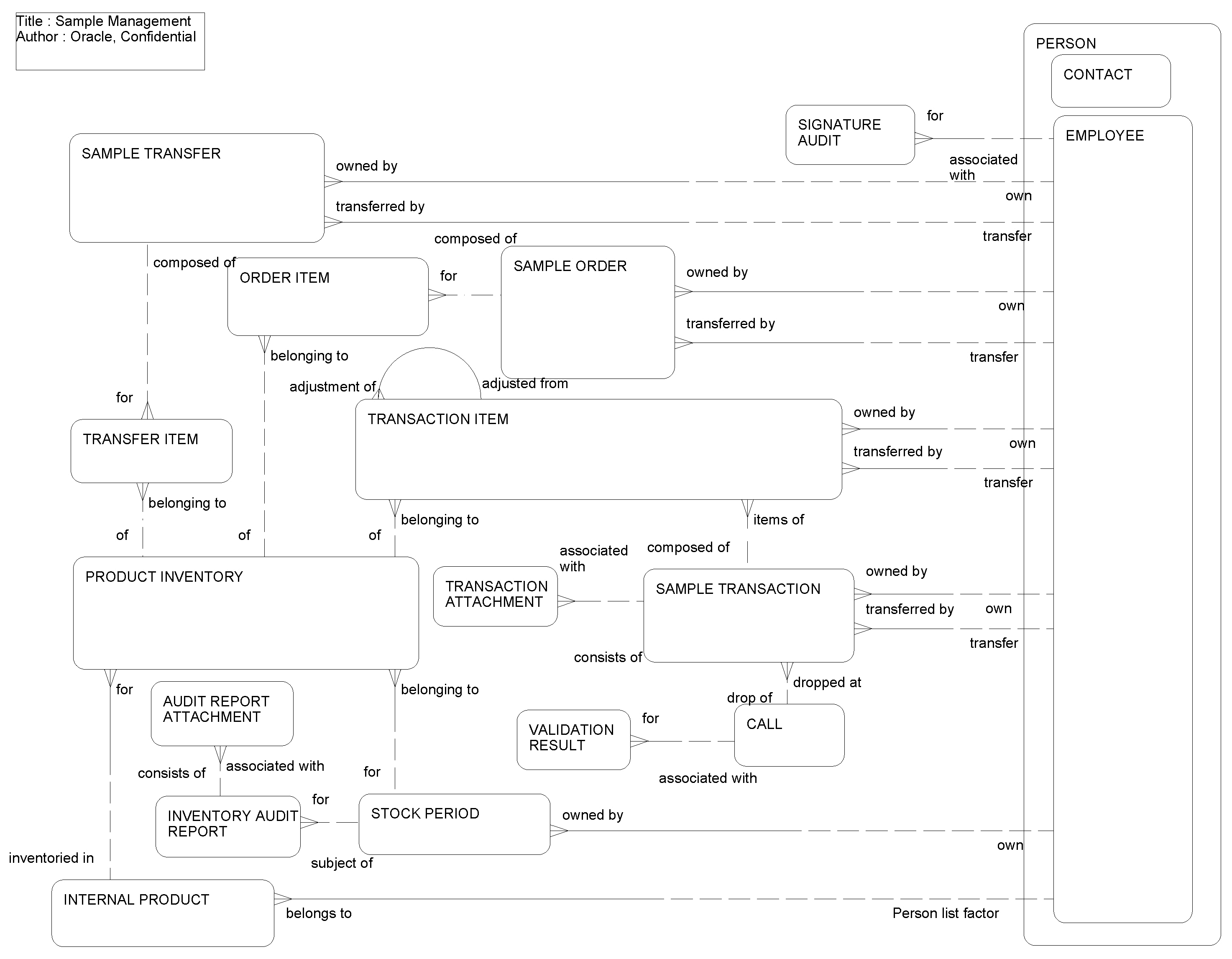
The following table lists the entities in this ERD and their corresponding tables.
| Entity | Table |
|---|---|
|
Audit Report Attachment |
S_INVADTRPT_ATT |
|
Call |
S_EVT_ACT |
|
Contact |
S_CONTACT, S_PARTY |
|
Employee |
S_EMP_PER, S_CONTACT, S_PARTY |
|
Internal Product |
S_PROD_INT |
|
Inventory Audit Report |
S_INV_AUDIT_RPT |
|
Order Item |
S_SMPL_TXN_ITEM |
|
Person |
S_CONTACT, S_PARTY, S_USER |
|
Product Inventory |
S_STOCK_EMP |
|
Sample Order |
S_SAMPLE_TXN |
|
Sample Transaction |
S_SAMPLE_TXN |
|
Sample Transfer |
S_SAMPLE_TXN |
|
Signature Audit |
S_EMP_AUDITSIGN |
|
Stock Period |
S_STK_PERD_EMP |
|
Transaction Attachment |
S_SMPL_TXN_ATT |
|
Transaction Item |
S_SMPL_TXN_ITEM |
|
Transfer Item |
S_SMPL_TXN_ITEM |
|
Validation Result |
S_VALDN_RESULT |