Clinical Study Management
This ERD (see the following figure) illustrates how clinical trials are managed. Each clinical trial starts with a protocol for a specific compound (product). Each protocol is conducted by sites and managed by site personnel. A protocol can have many versions and multiple protocols can roll up to a single program. Protocols can also roll up to regions. Subjects are screened and enrolled at protocol sites for specific protocol versions. Protocol sites are paid, based on the activities they complete. Visits and activities are generated for subjects based on templates defined for the protocol. The Clinical Research Associates perform site initiation activities for protocol sites and submit periodic trip reports. A protocol can also be associated with one or more projects. For a complete layout of projects, refer to Professional Services.
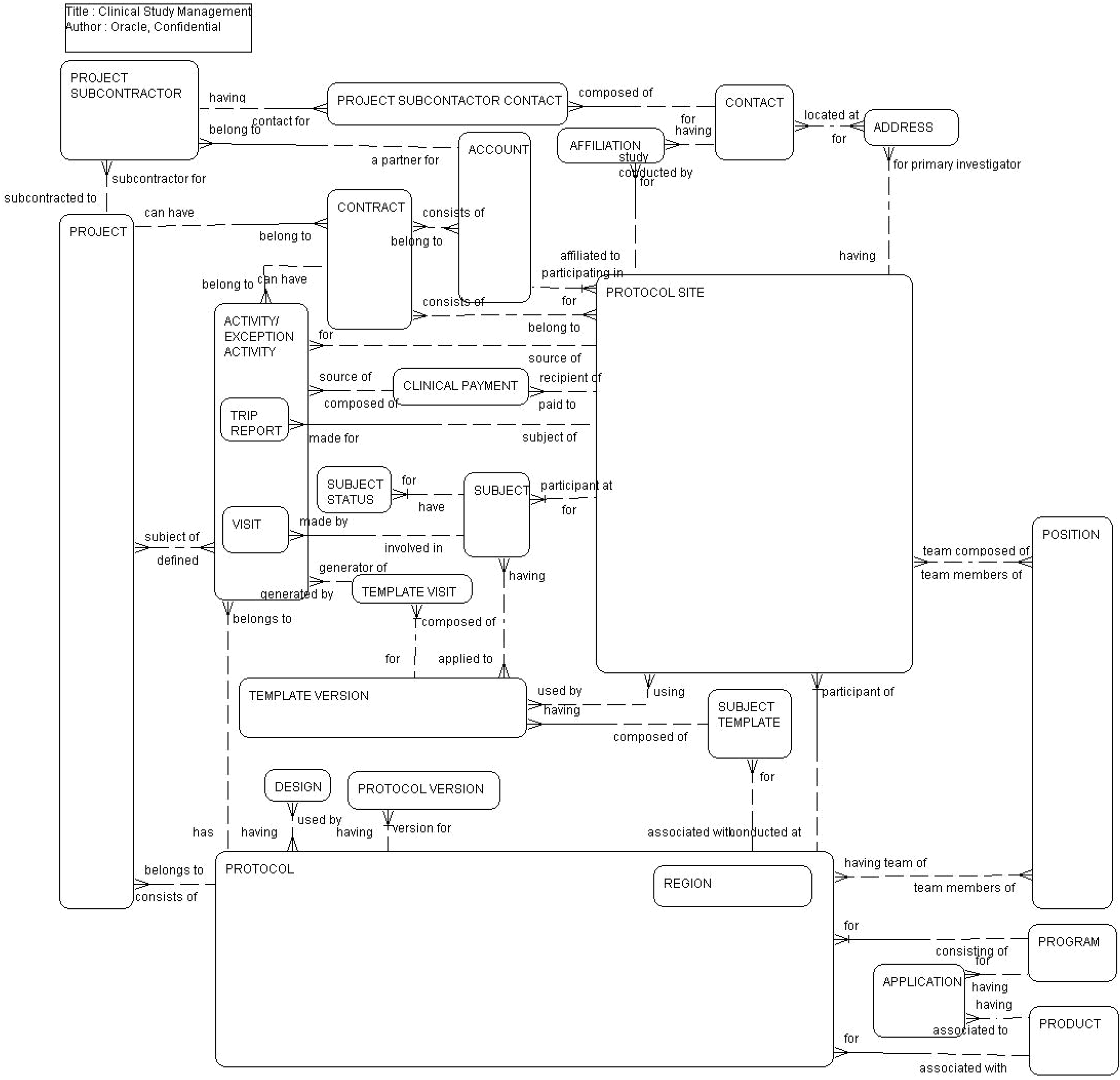
The following table lists the entities in this ERD and their corresponding tables.
| Entity | Table |
|---|---|
|
Account |
S_ORG_EXT, S_PARTY |
|
Activity |
S_EVT_ACT |
|
Address |
S_ADDR_PER |
|
Affiliation |
S_PTL_ST_CON_LS, S_PTL_STCONHIST |
|
Application |
S_CL_PGM_APP_LS |
|
Clinical Payment |
S_SRC_PAYMENT |
|
Contact |
S_CONTACT, S_PARTY |
|
Contract |
S_DOC_AGREE |
|
Design |
S_CL_DSGN_LS |
|
Exception Activity |
S_CL_ACT_EXC_LS |
|
Position |
S_POSTN |
|
Product |
S_PROD_INT |
|
Program |
S_CL_PGM_LS |
|
Project |
S_PROJ |
|
Project Subcontractor |
S_PROG_ORG |
|
Project Subcontractor Contact |
S_PROJ_ORG_CON |
|
Protocol |
S_CL_PTCL_LS |
|
Protocol Site |
S_PTCL_SITE_LS |
|
Subject |
S_CL_SUBJ_LS |
|
Subject Status |
S_CL_SUBJ_ST_LS |
|
Subject Template |
S_SUBJ_TMPL_LS |
|
Template Version |
S_SBJTMP_VER_LS |
|
Template Visit |
S_TMPL_PLANITEM |
|
Trip Report |
S_EVT_ACT |
|
Visit |
S_EVT_ACT |