Clinical Study Site Management
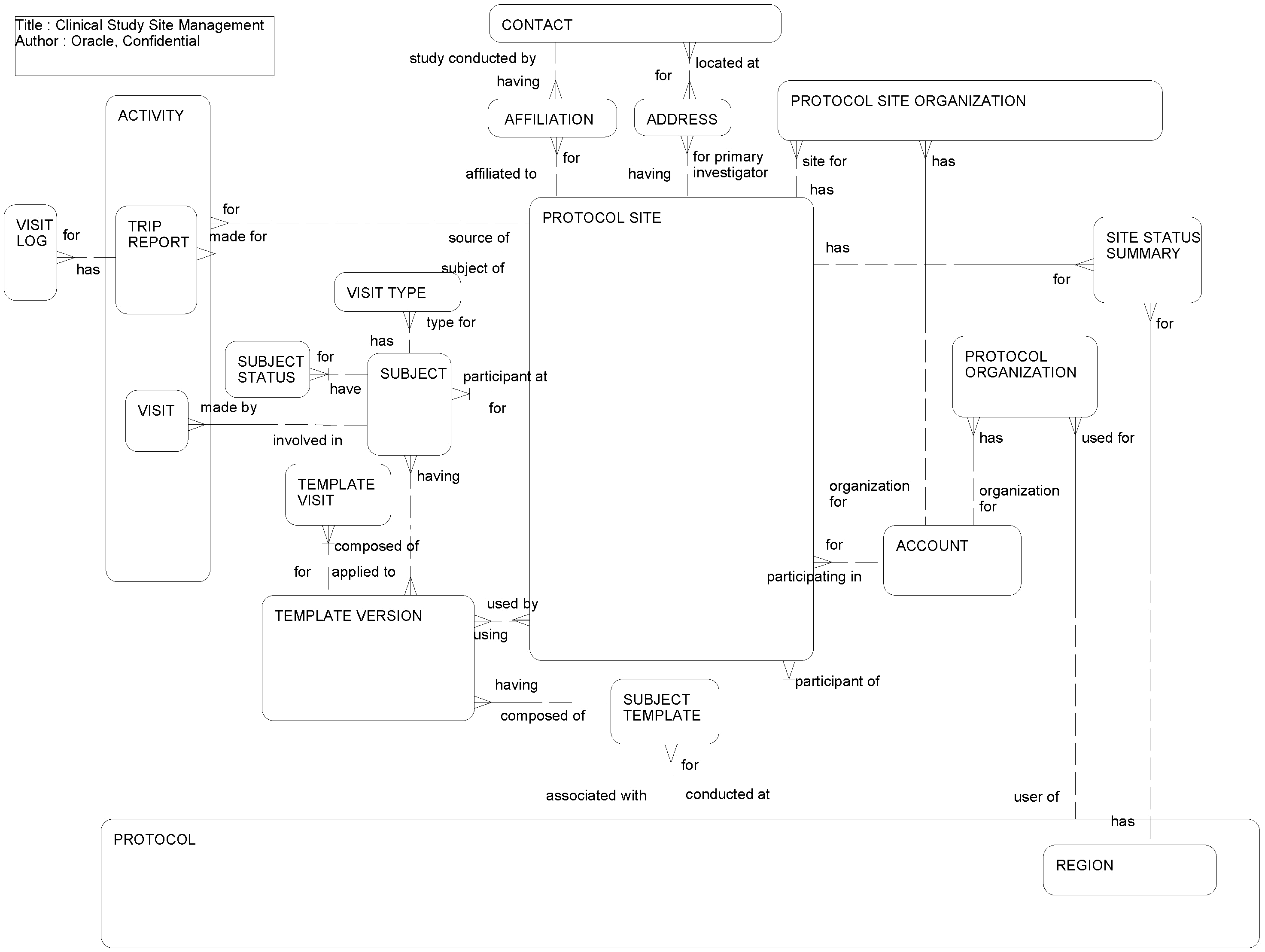
This ERD (see the following figure) is an extension of Clinical Study Management and illustrates:
-
Activities related to subjects participating in clinical studies and the list of visit types to be scheduled for this subject.
-
How site visit logs are maintained. Clinical Research Associates visit different targeted sites depending on research requirements and their visits are logged.
-
How organizations, such as vendors, sponsors, clinical research organizations, central laboratories, and institutional review boards, are associated with clinical protocol and clinical protocol sites.
-
How summaries of subject visits are organized by visit type and by protocol site.
The following table lists the entities in this ERD and their corresponding tables.
| Entity | Table |
|---|---|
|
Account |
S_ORG_EXT, S_PARTY |
|
Activity |
S_EVT_ACT |
|
Address |
S_ADDR_PER_S_CON_ADDR |
|
Affiliation |
S_PTCL_ST_CON_LS |
|
Contact |
S_CONTACT, S_PARTY |
|
Protocol |
S_CL_PTCL_LS |
|
Protocol Site |
S_PTCL_SITE_LS |
|
Protocol Organization |
S_PTCL_ORG_LS |
|
Protocol Site Organization |
S_PTCLST_ORG_LS |
|
Site Status Summary |
S_CL_SIT_ST_SUM |
|
Subject |
S_CL_SUBJ_LS |
|
Subject Status |
S_CL_SUBJ_ST_LS |
|
Subject Template |
S_SUBJ_TMPL_LS |
|
Subject Visit Type |
S_CL_SUBJVST_TP |
|
Template Version |
S_SBJTMP_VER_LS |
|
Template Visit |
S_TMPL_PLANITEM |
|
Visit |
S_EVT_ACT |
|
Visit Log |
S_SITE_VST_LOG |